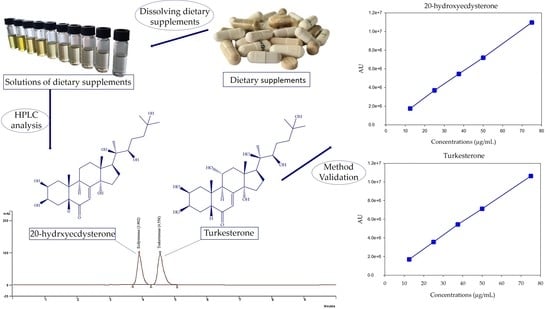
Development and Validation of High-Performance Liquid Chromatography for Identification and Quantification of Phytoecdysteroids Ecdysterone and Turkesterone in Dietary Supplements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. HPLC Analyses
2.3.1. Standard Solutions
2.3.2. Sample Solutions
2.3.3. Instrumentation
2.3.4. Method Development
2.3.5. Method Validation
3. Results
3.1. Method Development
3.2. Method Validation
- Linearity
- Accuracy and precision
- Limit of detection (LD) and limit of quantification (LQ)
- Robustness
- Stability
3.3. Method Application—Analysis of Dietary Supplements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dinan, L. Phytoecdysteroids: Biological Aspects. Phytochemistry 2001, 57, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, R.G.; Veskina, N.A.; Odinokov, V.N.; Benkovskaya, G.V.; Parfenova, L.V. Ecdysteroids: Isolation, Chemical Transformations, and Biological Activity. Phytochem. Rev. 2022, 21, 1445–1486. [Google Scholar] [CrossRef]
- Ecdybase (The Ecdysone Handbook)—A Free Online Ecdysteroids Database. Available online: https://ecdybase.org/ (accessed on 3 May 2023).
- Bajguz, A.; Bąkała, I.; Talarek, M. Chapter 5—Ecdysteroids in Plants and Their Pharmacological Effects in Vertebrates and Humans. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 45, pp. 121–145. [Google Scholar]
- Arif, Y.; Singh, P.; Bajguz, A.; Hayat, S. Phytoecdysteroids: Distribution, Structural Diversity, Biosynthesis, Activity, and Crosstalk with Phytohormones. Int. J. Mol. Sci. 2022, 23, 8664. [Google Scholar] [CrossRef] [PubMed]
- Kokoska, L.; Janovska, D. Chemistry and Pharmacology of Rhaponticum Carthamoides: A Review. Phytochemistry 2009, 70, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Mamarasulov, B.; Davranov, K.; Jabborova, D. Phytochemical, Pharmacological and Biological Properties of Ajuga Turkestanica (Rgl.) Brig (Lamiaceae). Ann. Phytomedicine 2020, 9, 44–57. [Google Scholar] [CrossRef]
- Todorova, V.; Ivanov, K.; Delattre, C.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S. Plant Adaptogens—History and Future Perspectives. Nutrients 2021, 13, 2861. [Google Scholar] [CrossRef] [PubMed]
- Báthori, M.; Zupkó, I.; Hunyadi, A.; Gácsné-Baitz, E.; Dinya, Z.; Forgó, P. Monitoring the Antioxidant Activity of Extracts Originated from Various Serratula Species and Isolation of Flavonoids from Serratula Coronata. Fitoterapia 2004, 75, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Odinokov, V.N.; Galyautdinov, I.V.; Nedopekin, D.V.; Khalilov, L.M.; Shashkov, A.S.; Kachala, V.V.; Dinan, L.; Lafont, R. Phytoecdysteroids from the Juice of Serratula Coronata L. (Asteraceae). Insect Biochem. Mol. Biol. 2002, 32, 161–165. [Google Scholar] [CrossRef]
- Guibout, L.; Mamadalieva, N.; Balducci, C.; Girault, J.-P.; Lafont, R. The Minor Ecdysteroids from Ajuga Turkestanica: Minor Ecdysteroids from Ajuga Turkestanic a. Phytochem. Anal. 2015, 26, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Wang, J.; Li, X.; Meng, D.; Li, X. Constituents of phytosterone in Cyanotis arachnoidea C.B.Clark. J. Shenyang Pharm. University 2001, 18, 263–265. [Google Scholar]
- Isenmann, E.; Ambrosio, G.; Joseph, J.F.; Mazzarino, M.; de la Torre, X.; Zimmer, P.; Kazlauskas, R.; Goebel, C.; Botrè, F.; Diel, P.; et al. Ecdysteroids as Non-Conventional Anabolic Agent: Performance Enhancement by Ecdysterone Supplementation in Humans. Arch. Toxicol. 2019, 93, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Tóth, N.; Szabó, A.; Kacsala, P.; Héger, J.; Zádor, E. 20-Hydroxyecdysone Increases Fiber Size in a Muscle-Specific Fashion in Rat. Phytomedicine 2008, 15, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Bathori, M.; Toth, N.; Hunyadi, A.; Marki, A.; Zador, E. Phytoecdysteroids and Anabolic-Androgenic Steroids—Structure and Effects on Humans. Curr. Med. Chem. 2008, 15, 75–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuvalov, O.; Kirdeeva, Y.; Fefilova, E.; Netsvetay, S.; Zorin, M.; Vlasova, Y.; Fedorova, O.; Daks, A.; Parfenyev, S.; Barlev, N. 20-Hydroxyecdysone Confers Antioxidant and Antineoplastic Properties in Human Non-Small Cell Lung Cancer Cells. Metabolites 2023, 13, 656. [Google Scholar] [CrossRef]
- Kraiem, S.; Al-Jaber, M.Y.; Al-Mohammed, H.; Al-Menhali, A.S.; Al-Thani, N.; Helaleh, M.; Samsam, W.; Touil, S.; Beotra, A.; Georgakopoulas, C.; et al. Analytical Strategy for the Detection of Ecdysterone and Its Metabolites in Vivo in UPA(+/+)-SCID Mice with Humanized Liver, Human Urine Samples, and Estimation of Prevalence of Its Use in Anti-doping Samples. Drug Test Anal. 2021, 13, 1341–1353. [Google Scholar] [CrossRef]
- Pálinkás, Z.; Békési, D.; Utczás, M. Quantitation of Ecdysterone and Targeted Analysis of WADA-Prohibited Anabolic Androgen Steroids, Hormones, and Metabolic Modulators in Ecdysterone-Containing Dietary Supplements. Separations 2023, 10, 242. [Google Scholar] [CrossRef]
- Haupt, O. Recent Advances in Doping Analysis (20). Proceedings of the Manfred-Donike-Workshop, 30th Cologne Workshop on Dope Analysis, 26 February–2nd March 2012; Sportverl Strauß: Köln, Germany, 2012. [Google Scholar]
- Das, N.; Mishra, S.K.; Bishayee, A.; Ali, E.S.; Bishayee, A. The Phytochemical, Biological, and Medicinal Attributes of Phytoecdysteroids: An Updated Review. Acta Pharm. Sin. B 2021, 11, 1740–1766. [Google Scholar] [CrossRef]
- Syrov, V.N.; Saatov, Z.; Sagdullaev, S.S.; Mamatkhanov, A.U. Study of the Structure—Anabolic Activity Relationship for Phytoecdysteroids Extracted from Some Plants of Central Asia. Pharm. Chem. J. 2001, 35, 667–671. [Google Scholar] [CrossRef]
- Cahlíková, L.; Macáková, K.; Chlebek, J.; Hošt’álková, A.; Kulhánková, A.; Opletal, L. Ecdysterone and Its Activity on Some Degenerative Diseases. Nat. Prod. Commun. 2011, 6, 1934578X1100600527. [Google Scholar] [CrossRef] [Green Version]
- Ferro, N.; Tacoronte, J.E.; Reinard, T.; Bultinck, P.; Montero, L.A. Structure–Activity Analysis on Ecdysteroids: A Structural and Quantum Chemical Approach Based on Two Biological Systems. J. Mol. Struct. Theochem. 2006, 758, 263–274. [Google Scholar] [CrossRef]
- Dinan, L. Ecdysteroid Structure-Activity Relationships. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Bioactive Natural Products (Part J); Elsevier: Amsterdam, The Netherlands, 2003; Volume 29, pp. 3–71. [Google Scholar]
- The WADA 2020 Monitoring Program. Available online: https://www.Wada-Ama.Org/Sites/Default/Files/Wada_2020_english_monitoring_program_.Pdf (accessed on 3 May 2023).
- The WADA 2023 Monitoring Program. Available online: https://www.Wada-Ama.Org/Sites/Default/Files/2022-09/2023list_monitoring_program_en_final_9_september_2022.Pdf (accessed on 3 May 2023).
- Parr, M.K.; Ambrosio, G.; Wuest, B.; Mazzarino, M.; De La Torre, X.; Sibilia, F.; Joseph, J.F.; Diel, P.; Botrè, F. Targeting the Administration of Ecdysterone in Doping Control Samples; Pharmacology and Toxicology: Tucson, AZ, USA, 2019. [Google Scholar]
- Grucza, K.; Wicka, M.; Drapała, A.; Kwiatkowska, D. Determination of Ecdysterone in Dietary Supplements and Spinach by Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry. Separations 2022, 9, 8. [Google Scholar] [CrossRef]
- Ecdysteroid-Containing Food Supplements from Cyanotis Arachnoidea on the European Market: Evidence for Spinach Product Counterfeiting|Scientific Reports. Available online: https://www.nature.com/articles/srep37322 (accessed on 4 May 2023).
- Timofeev, N.P.; Koksharov, A.V. Study of Leuzea Leuzea from Leaves: Results of 15 Years of Trials in Athletics. New Unconv. Plants Prospect. Use 2016, 12, 502–505. [Google Scholar]
- Wu, J.; Gao, L.; Shang, L.; Wang, G.; Wei, N.; Chu, T.; Chen, S.; Zhang, Y.; Huang, J.; Wang, J.; et al. Ecdysterones from Rhaponticum Carthamoides (Willd.) Iljin Reduce Hippocampal Excitotoxic Cell Loss and Upregulate MTOR Signaling in Rats. Fitoterapia 2017, 119, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhang, Q.; Liu, R.; Wang, Z.; Tang, N.; Liu, F.; Huang, G.; Jiang, X.; Gui, G.; Wang, L.; et al. Effects of 20-Hydroxyecdysone on Improving Memory Deficits in Streptozotocin-Induced Type 1 Diabetes Mellitus in Rat. Eur. J. Pharmacol. 2014, 740, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Krasutsky, A.G.; Cheremisinov, V.N. The Use of Levzey’s Extract to Increase the Efficiency of the Training Process in Fitness Clubs Students. In Proceedings of the Actual Problems of Biochemistry and Bioenergy of Sport of the XXI Century, Moscow, Russia, 10–26 April 2017; pp. 382–388. [Google Scholar]
- Garthe, I.; Maughan, R.J. Athletes and Supplements: Prevalence and Perspectives. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 126–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozhuharov, V.R.; Ivanov, K.; Ivanova, S. Dietary Supplements as Source of Unintentional Doping. BioMed. Res. Int. 2022, 2022, e8387271. [Google Scholar] [CrossRef]
- Vovk, I.; Glavnik, V. Chapter 21—Analysis of Dietary Supplements. In Instrumental Thin-Layer Chromatography; Poole, C.F., Ed.; Elsevier: Boston, MA, USA, 2015; pp. 589–635. ISBN 978-0-12-417223-4. [Google Scholar]
- Gafner, S.; Blumenthal, M.; Foster, S.; Cardellina, J.H.I.; Khan, I.A.; Upton, R. Botanical Ingredient Forensics: Detection of Attempts to Deceive Commonly Used Analytical Methods for Authenticating Herbal Dietary and Food Ingredients and Supplements. J. Nat. Prod. 2023, 86, 460–472. [Google Scholar] [CrossRef]
- Sharma, M.K.; Koppisetti, H.P.; Lohar, P.; Maheshwari, R.; Sengupta, P.; Tekade, R.K. Chapter 23—Understanding the Connection between Dietary Supplementation and Inadvertent Doping. In Essentials of Pharmatoxicology in Drug Research; Tekade, R., Ed.; Advances in Pharmaceutical Product Development and Research; Academic Press: Cambridge, MA, USA, 2023; Volume 1, pp. 599–623. ISBN 978-0-443-15840-7. [Google Scholar]
- Sadia, M.; Mahmood, A.; Ibrahim, M.; Irshad, M.K.; Quddusi, A.H.A.; Bokhari, A.; Mubashir, M.; Chuah, L.F.; Show, P.L. Microplastics Pollution from Wastewater Treatment Plants: A Critical Review on Challenges, Detection, Sustainable Removal Techniques and Circular Economy. Environ. Technol. Innov. 2022, 28, 102946. [Google Scholar] [CrossRef]
- EMA ICH Q2(R2) Validation of Analytical Procedures—Scientific Guideline. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 7 December 2022).
- Poojari, R. Phytochemical Fingerprinting, Cytotoxic, Antimicrobial, Antitubercular, Antimycotic Potentials of Sida Rhombifolia Subsp. Retusa and Embelia Tsjeriam-Cottam. Asia Pac. Life Sci. Compend. 2011, 4, 201–214. [Google Scholar]
- Liktor-Busa, E.; Báthori, M. Analysis of the Ecdysteroid Profile of Serratula Wolffii Roots. Ph.D. Thesis, University of Szeged, Szeged, Hungary, 2008. [Google Scholar]
- Głazowska, J.; Kamiński, M.M.; Kamiński, M. Chromatographic Separation, Determination and Identification of Ecdysteroids: Focus on Maral Root (Rhaponticum Carthamoides, Leuzea Carthamoides). J. Sep. Sci. 2018, 41, 4304–4314. [Google Scholar] [CrossRef]
- Dinan, L.; Harmatha, J.; Lafont, R. Chromatographic Procedures for the Isolation of Plant Steroids. J. Chromatogr. A 2001, 935, 105–123. [Google Scholar] [CrossRef]
- Ambrosio, G.; Wirth, D.; Joseph, J.F.; Mazzarino, M.; de la Torre, X.; Botrè, F.; Parr, M.K. How Reliable Is Dietary Supplement Labelling?-Experiences from the Analysis of Ecdysterone Supplements. J. Pharm. Biomed. Anal. 2020, 177, 112877. [Google Scholar] [CrossRef]
- Lafont, R.; Dinan, L. Practical Uses for Ecdysteroids in Mammals Including Humans: And Update. J. Insect Sci. 2003, 3, 7. [Google Scholar] [CrossRef]
- Petkova-Gueorguieva, E.S.; Getov, I.N.; Ivanov, K.V.; Ivanova, S.D.; Gueorguiev, S.R.; Getova, V.I.; Mihaylova, A.A.; Madzharov, V.G.; Staynova, R.A. Regulatory Requirements for Food Supplements in the European Union and Bulgaria. Folia. Med. (Plovdiv.) 2019, 61, 41–48. [Google Scholar] [CrossRef]
- Lam, M.; Khoshkhat, P.; Chamani, M.; Shahsavari, S.; Dorkoosh, F.A.; Rajabi, A.; Maniruzzaman, M.; Nokhodchi, A. In-Depth Multidisciplinary Review of the Usage, Manufacturing, Regulations & Market of Dietary Supplements. J. Drug Deliv. Sci. Technol. 2022, 67, 102985. [Google Scholar] [CrossRef]
- Tsitsimpikou, C.; Tsamis, G.D.; Siskos, P.A.; Spyridaki, M.-H.E.; Georgakopoulos, C.G. Study of Excretion of Ecdysterone in Human Urine. Rapid Commun. Mass Spectrom. 2001, 15, 1796–1801. [Google Scholar] [CrossRef]
- De Cock, K.J.S.; Delbeke, F.T.; Van Eenoo, P.; Desmet, N.; Roels, K.; De Backer, P. Detection and Determination of Anabolic Steroids in Nutritional Supplements. J. Pharm. Biomed. Anal. 2001, 25, 843–852. [Google Scholar] [CrossRef]
- Parys, W.; Dołowy, M.; Pyka-Pająk, A. Significance of Chromatographic Techniques in Pharmaceutical Analysis. Processes 2022, 10, 172. [Google Scholar] [CrossRef]
- Parys, W.; Dołowy, M.; Pyka-Pająk, A. Current Strategies for Studying the Natural and Synthetic Bioactive Compounds in Food by Chromatographic Separation Techniques. Processes 2021, 9, 1100. [Google Scholar] [CrossRef]
- Munir, M.; Saeed, M.; Ahmad, M.; Waseem, A.; Alsaady, M.; Asif, S.; Ahmed, A.; Shariq Khan, M.; Bokhari, A.; Mubashir, M.; et al. Cleaner Production of Biodiesel from Novel Non-Edible Seed Oil (Carthamus Lanatus L.) via Highly Reactive and Recyclable Green Nano CoWO3@rGO Composite in Context of Green Energy Adaptation. Fuel 2023, 332, 126265. [Google Scholar] [CrossRef]
- Thiem, B.; Kikowska, M.; Maliński, M.P.; Kruszka, D.; Napierała, M.; Florek, E. Ecdysteroids: Production in Plant in Vitro Cultures. Phytochem. Rev. 2017, 16, 603–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinan, L.; Balducci, C.; Guibout, L.; Lafont, R. Database of Human Food Plants and Whether They Have Been Assessed for the Presence or Absence of Ecdysteroids. Available online: https://ecdybase.org/ecdysteroids_in_food_plants.pdf (accessed on 22 May 2023).
- The Wada 2023 Prohibitet List. Available online: https://www.Wada-Ama.Org/Sites/Default/Files/2023-05/2023list_en_final_9_september_2022.Pdf (accessed on 22 May 2023).




| Concentration (μg/mL) | Mean (μg/mL) ± SD | Recovery % | CV% |
|---|---|---|---|
| 20-hydroxyecdysterone | |||
| 50 | 49.93 ± 0.18 | 99.85 | 0.36 |
| 25 | 25.06 ± 0.14 | 100.25 | 0.55 |
| 16 | 16.10 ± 0.08 | 100.65 | 0.51 |
| Turkesterone | |||
| 50 | 50.16 ± 0.27 | 100.32 | 0.54 |
| 25 | 24.93 ± 0.11 | 99.73 | 0.44 |
| 16 | 16.02 ± 0.19 | 100.11 | 1.19 |
| Concentration (μg/mL) | Intra-Day Precision | Inter-Day Precision | ||||
|---|---|---|---|---|---|---|
| Mean (μg/mL) ± SD | Standard Error | CV% | Mean (μg/mL) ± SD | Standard Error | CV% | |
| 20-hydroxyecdysterone | ||||||
| 50 | 49.94 ± 0.18 | 0.07 | 0.35 | 49.99 ± 0.23 | 0.09 | 0.47 |
| 25 | 25.08 ± 0.15 | 0.06 | 0.61 | 25.02 ± 0.08 | 0.03 | 0.33 |
| 16 | 16.08 ± 0.07 | 0.03 | 0.41 | 15.99 ± 0.05 | 0.02 | 0.30 |
| Turkesterone | ||||||
| 50 | 50.03 ± 0.08 | 0.03 | 0.15 | 50.03 ± 0.08 | 0.03 | 0.15 |
| 25 | 25.02 ± 0.07 | 0.03 | 0.26 | 25.04 ± 0.05 | 0.02 | 0.19 |
| 16 | 15.99 ± 0.07 | 0.03 | 0.42 | 16.01 ± 0.09 | 0.04 | 0.59 |
| Sample № | Country of Origin of the Manufacturer | Sample Type | Product Description | ES Content (mg/g) | Determined ES Content (mg/Capsule or Tablet) | TS Content (mg/g) | Determined TS Content (mg/Capsule or Tablet) | ES/TS Labeled Content | Labeled Extract Content (mg/Capsule or Tablet) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Czech Republic | Capsule | C. arachnoidea extract | 188.07 mg/g | 87.00 mg | 38.33 mg/g | 17.73 mg | 90% ecdysterone | 300 mg |
| 2 | American brand; where produced not labeled | Capsule | R. carthamoides extract | 302.24 mg/g | 91.88 mg | 1.06 mg/g | 0.32 mg | Not labeled | 250 mg |
| 3 | Bulgaria | Capsule | β-ecdysterone from R. carthamoides extract | 44.16 mg/g | 19.30 mg | 2 mg/g | 0.80 mg | 95% ecdysterone | 245 mg |
| 4 | American brand; where produced not labeled | Capsule | R. carthamoides extract | 232.71 mg/g | 79.82 mg | 57.73 mg/g | 19.80 mg | 80% ecdysterone | 250 mg |
| 5 | Bulgaria | Tablet | R. carthamoides extract | 17.52 mg/g | 5.29 mg | 0.75 mg/g | 2.48 mg | Not labeled | 200 mg |
| 6 | Bulgaria | Tablet | R. carthamoides extract | 151.17 mg/g | 14.80 mg | 12.97 mg/g | 1.27 mg | 15 mg | 35 mg |
| 7 | Bulgaria | Tablet | R. carthamoides extract | 125.06 mg/g | 16.32 mg | 0.92 mg/g | 0.12 mg | 16.5 mg | 40 mg |
| 8 | Czech Republic | Capsule | R. carthamoides extract | 61.65 mg/g | 35. 14 mg | - | - | 300 mg | 330 mg |
| 9 | Sweden | Capsule | A. turkestanica extract + Ginseng and Astragal extract | 0.87 mg/g | 0.56 mg | 80.14 mg/g | 51.77 mg | Min. 10% turkesterone | 500 mg |
| 10 | Bulgaria | Capsule | A. turkestanica extract | 178.27 mg/g | 47.26 mg | 1.06 mg/g | 0.64 mg | 10 % turkesterone | 500 mg |
| 11 | American brand; where produced not labeled | Capsule | A. turkestanica extract | 40.35 mg/g | 19.64 mg | 12.80 mg/g | 6.23 mg | 10 % turkesterone | 500 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, V.; Ivanov, K.; Karcheva-Bahchevanska, D.; Ivanova, S. Development and Validation of High-Performance Liquid Chromatography for Identification and Quantification of Phytoecdysteroids Ecdysterone and Turkesterone in Dietary Supplements. Processes 2023, 11, 1786. https://doi.org/10.3390/pr11061786
Todorova V, Ivanov K, Karcheva-Bahchevanska D, Ivanova S. Development and Validation of High-Performance Liquid Chromatography for Identification and Quantification of Phytoecdysteroids Ecdysterone and Turkesterone in Dietary Supplements. Processes. 2023; 11(6):1786. https://doi.org/10.3390/pr11061786
Chicago/Turabian StyleTodorova, Velislava, Kalin Ivanov, Diana Karcheva-Bahchevanska, and Stanislava Ivanova. 2023. "Development and Validation of High-Performance Liquid Chromatography for Identification and Quantification of Phytoecdysteroids Ecdysterone and Turkesterone in Dietary Supplements" Processes 11, no. 6: 1786. https://doi.org/10.3390/pr11061786
APA StyleTodorova, V., Ivanov, K., Karcheva-Bahchevanska, D., & Ivanova, S. (2023). Development and Validation of High-Performance Liquid Chromatography for Identification and Quantification of Phytoecdysteroids Ecdysterone and Turkesterone in Dietary Supplements. Processes, 11(6), 1786. https://doi.org/10.3390/pr11061786