Defatting and Defatted Peanuts: A Critical Review on Methods of Oil Extraction and Consideration of Solid Matrix as a By-Product or Intended Target
Abstract
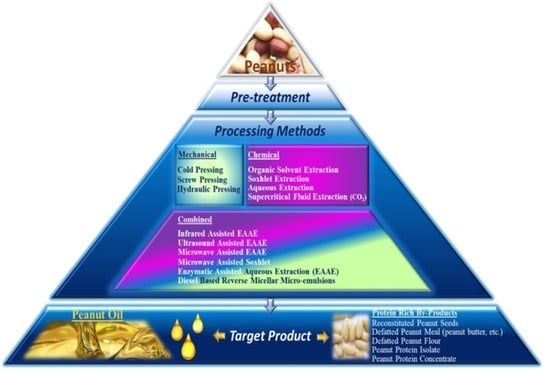
:1. Introduction
2. Mechanical Methods of Oil Extraction from Peanuts
2.1. Extrusion and Screw Pressing
2.2. Cold Pressing
2.3. Hydraulic Pressing
3. Chemical Methods of Oil Extraction from Peanuts
3.1. Organic Solvent Extraction
3.2. Aqueous Extraction
3.3. Supercritical Fluid CO2
4. Combined Methods of Oil Extraction from Peanuts
4.1. Enzyme-Assisted Aqueous Extraction
4.2. The Applications of Microwave-, Infrared-, and Ultrasound-Assisted Oil Extraction
4.3. Other Combined Methods for Oil Extraction
5. Advantages and Disadvantages of the Oil Extraction Methods from Peanuts
5.1. Mechanical Methods
5.2. Chemical Methods
5.3. Combined Methods
6. Other Technologies
7. Peanut Proteins Valorization
8. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | AFLATOXIN |
| AEP | Aqueous Extraction Processing |
| CNS | Central Nervous System |
| D.B. | Dry Basis |
| PDPM | Partially Defatted Peanut Meal |
| EAAE | Enzyme-Assisted Aqueous Extraction |
| IR | Infrared Radiation |
| IVDV | Intensification of Vaporization by Decompression to the Vacuum |
| MAEE | Microwave-Assisted Enzymatic Extraction |
| MEPSI | Mechanical Expression Preserving Shape Integrity |
| MF | Microfiltration |
| MIS | Microwave Integrated Soxhlet |
| POB | Peanut Oil Body |
| OSI | Oxidative Stability Index |
| P | Pressure |
| PDPF | Partially Defatted Peanut Flour |
| PPI | Peanut Protein Isolate |
| PPC | Peanut Protein Concentrate |
| PV | Peroxide Value |
| RPM | Round Per Minute |
| RSA | Radical Scavenging Activity |
| RSM | Response surface methodology |
| SC-CO2 | Supercritical CO2 |
| SCP | Semi-Continuous Process |
| SFE | Supercritical Fluid Extraction |
| T | Temperature |
| UF | Ultrafiltration |
| VOC | Volatile Organic Compound |
| W | Water Content |
| W.B. | Water Basis |
References
- Nader, J.; Afif, C.; Louka, N. A new eco-friendly defatting process of peanuts by mechanical expression preserving structure integrity (MEPSI). In Proceedings of the International Conference on Renewable Energies for Developing Countries 2014, Beirut, Lebanon, 26–27 November 2014; pp. 54–59. [Google Scholar] [CrossRef]
- Nader, J.; Fawaz, N.; Afif, C.; Louka, N. A novel process for preparing low-fat peanuts: Optimization of the oil extraction yield with limited structural and organoleptic damage. Food Chem. 2016, 197, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Nader, J.; Afif, C.; Louka, N. Study of physiological and textural properties of roasted peanuts defatted by an innovative oil extraction process. Correlation with consumer evaluation. Innov. Food Sci. Emerg. Technol. 2016, 33, 450–461. [Google Scholar] [CrossRef]
- Nader, J.; Louka, N. Development of a novel technology entitled “Intensification of Vaporization by Decompression to the Vacuum” (IVDV) for reconstitution and texturing of partially defatted peanuts. Innov. Food Sci. Emerg. Technol. 2018, 45, 455–466. [Google Scholar] [CrossRef]
- Özcan, M.; Seven, S. Análisis físico-químicas y composición de ácidos grasos del aceite de maní maní y mantequilla de maní. Grasas 2003, 54, 12–18. [Google Scholar]
- Ji, J.; Liu, Y.; Wang, D. Comparison of de-skin pretreatment and oil extraction on aflatoxins, phthalate esters, and polycyclic aromatic hydrocarbons in peanut oil. Food Control 2020, 118, 107365. [Google Scholar] [CrossRef]
- Yu, S.; Pan, L.; Yang, Q.; Min, P.; Ren, Z.; Zhang, H. Comparison of the Δ12 fatty acid desaturase gene between high-oleic and normal-oleic peanut genotypes. J. Genet. Genom. 2008, 35, 679–685. [Google Scholar] [CrossRef]
- Nader, J.; Afif, C.; Louka, N. Expansion of partially defatted peanuts by a new texturizing process called “Intensification of Vaporization by Decompression to the Vacuum” (IVDV). Innov. Food Sci. Emerg. Technol. 2017, 41, 179–187. [Google Scholar] [CrossRef]
- Lovejoy, J.C. The impact of nuts on diabetes and diabetes risk. Curr. Diab. Rep. 2005, 5, 379–384. [Google Scholar] [CrossRef]
- Sabaté, J.; Ang, Y. Nuts and health outcomes: New epidemiologic evidence. Am. J. Clin. Nutr. 2009, 89, 1643–1648. [Google Scholar] [CrossRef]
- Çiftçi, S.; Suna, G. Functional components of peanuts (Arachis hypogaea L.) and health benefits: A review. Futur. Foods 2022, 5, 100140. [Google Scholar] [CrossRef]
- Griel, A.E.; Eissenstat, B.; Kris-Etherton, P.M.; Hsieh, G.; Juturu, V. Improved Diet Quality with Peanut Consumption. J. Am. Coll. Nutr. 2004, 23, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Cämmerer, B.; Kroh, L.W. Shelf life of linseeds and peanuts in relation to roasting. LWT 2009, 42, 545–549. [Google Scholar] [CrossRef]
- Suchoszek-Łukaniuk, K.; Jaromin, A.; Korycińska, M.; Kozubek, A. Health Benefits of Peanut (Arachis hypogaea L.) Seeds and Peanut Oil Consumption. In Nuts and Seeds in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2011; pp. 873–880. [Google Scholar] [CrossRef]
- Rehrah, D.; Ahmedna, M.; Goktepe, I.; Yu, J. Extrusion parameters and consumer acceptability of a peanut-based meat analogue. Int. J. Food Sci. Technol. 2009, 44, 2075–2084. [Google Scholar] [CrossRef]
- Riaz, M.N.; Cheewapramong, P. Characterization of partially defatted peanut flour using dry extruder and screw pressing. Int. J. Food Prop. 2009, 12, 427–437. [Google Scholar] [CrossRef]
- Suri, K.; Singh, B.; Kaur, A.; Singh, N. Impact of roasting and extraction methods on chemical properties, oxidative stability and Maillard reaction products of peanut oils. J. Food Sci. Technol. 2019, 56, 2436–2445. [Google Scholar] [CrossRef]
- Bargale, P.C.; Ford, R.J.; Sosulski, F.W.; Wulfsohn, D.; Irudayaraj, J. Mechanical Oil Expression from Extruded Soybean Samples. JAOCS J. Am. Oil Chem. Soc. 1999, 76, 223–229. [Google Scholar] [CrossRef]
- Çakaloğlu, B.; Özyurt, V.H.; Ötleş, S. Cold press in oil extraction. A review. Ukr. Food J. 2018, 7, 640–654. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Zhang, Q. Research on exacting oil technique of peanut with low temperature. In Proceedings of the 2017 7th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2017), Ya’an, China, 10–11 May 2017; Volume 136, pp. 126–130. [Google Scholar]
- Shin, S.; Xuan, C.; Tyug, S. Cold Pressed Peanut (Arachis hypogaea L.) Oil; Elsevier Inc.: Kuala Lumpur, Malaysia, 2020; ISBN 9780128181881. [Google Scholar]
- Nader, J.; Afif, C.; Louka, N. Impact of a novel partial defatting technology on oxidative stability and sensory properties of peanut kernels. Food Chem. 2021, 334, 127581. [Google Scholar] [CrossRef]
- Mrad, R.; El Rammouz, R.; Maroun, R.G.; Louka, N. Effect of intensification of vaporization by decompression to the vacuum as a pretreatment for roasting australian chickpea: Multiple optimization by response surface methodology of chemical, textural and color parameters. J. Food Qual. 2015, 38, 139–152. [Google Scholar] [CrossRef]
- Mrad, R.; Debs, E.; Saliba, R.; Maroun, R.G.; Louka, N. Multiple optimization of chemical and textural properties of roasted expanded purple maize using response surface methodology. J. Cereal Sci. 2014, 60, 397–405. [Google Scholar] [CrossRef]
- Louka, N. Maîtrise de la Qualité des Produits Agro-Alimentaires Séchés: Modification Texturale et Réduction du coût Énergétique par Détente Instantanée Contrôlée “DIC’’ vers le vide: Conception et Réalisation d’un Nouveau Procédé Industriel. Ph.D. Dissertation, Université de technologie de Compiègne, Compiègne, France, 1996. [Google Scholar]
- Johnson, L.A.; Lusas, E.W. Comparison of alternative solvents for oils extraction. J. Am. Oil Chem. Soc. 1983, 60, 229–242. [Google Scholar] [CrossRef]
- Sharma, A.; Khare, S.K.; Gupta, M.N. Enzyme-assisted aqueous extraction of peanut oil. JAOCS J. Am. Oil Chem. Soc. 2002, 79, 215–218. [Google Scholar] [CrossRef]
- Jiang, L.; Hua, D.; Wang, Z.; Xu, S. Aqueous enzymatic extraction of peanut oil and protein hydrolysates. Food Bioprod. Process. 2010, 88, 233–238. [Google Scholar] [CrossRef]
- Wang, Y.; Han, F.; Wang, Z.; Cheng, S. Aqueous Enzymatic Extraction of Oil and Protein Hydrolysates from Peanut. Food Sci. Technol. Res. 2008, 14, 533–540. [Google Scholar] [CrossRef]
- Tu, J.; Wu, W. An advanced pilot method of separating peanut oils with high quality based on aqueous extraction. Sep. Sci. Technol. 2020, 55, 739–751. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Li, P.; Jiang, Z.; Yang, R. Isolation of peanut protein aggregates using aqueous extraction processing combined with membrane separation. Int. J. Food Sci. Technol. 2020, 55, 3203–3214. [Google Scholar] [CrossRef]
- Haji Heidari, S.; Taghian Dinani, S. The Study of Ultrasound-Assisted Enzymatic Extraction of Oil From Peanut Seeds Using Response Surface Methodology. Eur. J. Lipid Sci. Technol. 2018, 120, 1700252. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, L.; Sui, X.; Qi, B.; Han, Z. The study of ultrasonic-assisted aqueous enzymatic extraction of oil from peanut by response surface method. Procedia Eng. 2011, 15, 4653–4660. [Google Scholar] [CrossRef]
- Deng, B.X.; Li, B.; Li, X.D.; Zaaboul, F.; Jiang, J.; Li, J.W.; Liu, Y.F. Using Short-Wave Infrared Radiation to Improve Aqueous Enzymatic Extraction of Peanut Oil: Evaluation of Peanut Cotyledon Microstructure and Oil Quality. Eur. J. Lipid Sci. Technol. 2018, 120, 1700285. [Google Scholar] [CrossRef]
- Liu, W.; Xiao, B.; Yang, G.; Bi, Y.; Chen, F. Rapid Salt-Assisted Microwave Demulsification of Oil-Rich Emulsion Obtained by Aqueous Enzymatic Extraction of Peanut Seeds. Eur. J. Lipid Sci. Technol. 2020, 122, 1900120. [Google Scholar] [CrossRef]
- Idrus, N.F.M.; Yang, T.A. Comparison between roasting by superheated steam and by convection on changes in colour, texture and microstructure of peanut (Arachis hypogaea). Food Sci. Technol. Res. 2012, 18, 515–524. [Google Scholar] [CrossRef]
- Google Scholar. Available online: https://scholar.google.com/ (accessed on 1 February 2020).
- ScienceDirect. Available online: https://www.sciencedirect.com/ (accessed on 1 February 2020).
- Web of Science. Available online: https://access.clarivate.com/login?app=wos&alternative=true&shibShireURL=https:%2F%2Fwww.webofknowledge.com%2F%3Fauth%3DShibboleth&shibReturnURL=https:%2F%2Fwww.webofknowledge.com%2F&roaming=true (accessed on 2 February 2020).
- Wiley Online Library (Modern Language Association). Available online: https://www.mla.org/Publications/MLA-International-Bibliography/Free-Online-Course?gclid=EAIaIQobChMI3u-_0YGggAMV6otoCR0M2QesEAAYASAAEgL-MvD_BwE (accessed on 2 February 2020).
- ResearchGate. Available online: https://www.researchgate.net/ (accessed on 1 February 2020).
- Espacenet. Available online: https://worldwide.espacenet.com/ (accessed on 3 February 2020).
- Google Patents. Available online: https://patents.google.com/ (accessed on 3 February 2020).
- Rajha, H.N.; Azoury, J.; Debs, E.; El Azzi, E.; Louka, N. Optimization of peanuts’ defatting using Ired- Irrad®, a newly-patented green and low-cost technology. In Proceedings of the 2020 5th International Conference on Renewable Energies for Developing Countries (REDEC), Marrakech, Morocco, 29–30 June 2020; pp. 2–5. [Google Scholar] [CrossRef]
- Nader, J.; Afif, C.; Louka, N. Color and texture of low-calorie peanuts as affected by a new oil extraction process named “Mechanical Expression Preserving Shape Integrity” (MEPSI). J. Food Sci. Technol. 2016, 53, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Mridula, D.; Saha, D.; Gupta, R.K.; Bhadwal, S.; Arora, S.; Kumar, S.R. Oil Expelling from Dehulled De-Skinned Groundnut Kernel using Screw Press: Optimization of Process Parameters and Physico-chemical Characteristics. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1101–1115. [Google Scholar] [CrossRef]
- Olajide, J.O.; Igbeka, J.C.; Afolabi, T.J.; Emiola, O.A. Prediction of oil yield from groundnut kernels in an hydraulic press using artificial neural network (ANN). J. Food Eng. 2007, 81, 643–646. [Google Scholar] [CrossRef]
- Olajide, J. Optimization of Oil Yield from Groundnut Kernel (Arachis hypogeae) in an Hydraulic Press Using Response Surface Methodology. J. Sci. Res. Rep. 2014, 3, 1916–1926. [Google Scholar] [CrossRef]
- Nikmaram, N.; Leong, S.Y.; Koubaa, M.; Zhu, Z.; Barba, F.J.; Greiner, R.; Oey, I.; Roohinejad, S. Effect of extrusion on the anti-nutritional factors of food products: An overview. Food Control 2017, 79, 62–73. [Google Scholar] [CrossRef]
- Bhuiya, M.M.K.; Rasul, M.; Khan, M.; Ashwath, N.; Rahman, M. Comparison of oil extraction between screw press and solvent (n-hexane) extraction technique from beauty leaf (Calophyllum inophyllum L.) feedstock. Ind. Crops Prod. 2020, 144, 112024. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I. Peanut protein concentrate: Production and functional properties as affected by processing. Food Chem. 2007, 103, 121–129. [Google Scholar] [CrossRef]
- Konuskan, D.B.; Arslan, M.; Oksuz, A. Physicochemical properties of cold pressed sunflower, peanut, rapeseed, mustard and olive oils grown in the Eastern Mediterranean region. Saudi J. Biol. Sci. 2019, 26, 340–344. [Google Scholar] [CrossRef]
- Mutegi, C.K.; Ngugi, H.K.; Hendriks, S.L.; Jones, R.B. Prevalence and factors associated with aflatoxin contamination of peanuts from Western Kenya. Int. J. Food Microbiol. 2009, 130, 27–34. [Google Scholar] [CrossRef]
- Yin, Y.N.; Yan, L.Y.; Jiang, J.H.; Ma, Z.H. Biological control of aflatoxin contamination of crops. J. Zhejiang Univ. Sci. B 2008, 9, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Louka, N.; Nader, J. Treatment Process of Biological Products «Mechanical Pressing Extraction Preserving Product Integrity (MPEPPI)» Aiming at Modifying Their Lipid Content and Their Texture. Settings and Methods for the Implementation of Such Process. LB Pat LB-10492L, November 2014. [Google Scholar]
- Louka, N.; Nader, J.; Roufayel, M.; Kazzi, M.; El-Azzi, E. Seeds and Nuts Defatted by Pressing and Reconstituted by Methods Preserving Their Appearance and Organoleptic Properties. LB Pat LB-10493L, November 2014. [Google Scholar]
- Putra, N.R.; Yunus, M.A.C.; Ruslan, M.S.H.; Idham, Z.; Idrus, F.N. Comparison extraction of peanut skin between CO2 supercritical fluid extraction and soxhlet extraction in term of oil yield and catechin. Pertanika J. Sci. Technol. 2018, 26, 799–810. [Google Scholar]
- Chanvrier, H.; Jakubczyk, E.; Gondek, E.; Gumy, J.C. Insights into the texture of extruded cereals: Structure and acoustic properties. Innov. Food Sci. Emerg. Technol. 2014, 24, 61–68. [Google Scholar] [CrossRef]
- Chiou, R.Y.Y.; Yu, Z.R.; Wu, P.Y.; Chen, W.; Weng, Y.M. Partial defatting of roasted peanut meals and kernels by supercritical CO2 using semicontinuous and intermittently depressurized processes. J. Agric. Food Chem. 1996, 44, 574–578. [Google Scholar] [CrossRef]
- Fornasari, C.H.; Secco, D.; Santos, R.F.; da Silva, T.R.B.; Galant Lenz, N.B.; Tokura, L.K.; Lenz, M.L.; de Souza, S.N.M.; Zanão Junior, L.A.; Gurgacz, F. Efficiency of the use of solvents in vegetable oil extraction at oleaginous crops. Renew. Sustain. Energy Rev. 2017, 80, 121–124. [Google Scholar] [CrossRef]
- Anand, A.; Kaushal, M.; Vaidya, D.; Gupta, A. Supercritical fluid extraction as a novel technology for extraction of bioactive compounds: A review. Pharma Innov. J. 2022, 11, 2253–2262. [Google Scholar]
- Putra, N.R.; Hazim, A.; Aziz, A.; Idham, Z.; Syafiq, M.; Ruslan, H.; Azizi, M.; Yunus, C. Diffusivity optimization of supercritical carbon dioxide extraction with co-solvent-ethanol from peanut skin. Malays. J. Fundam. Appl. Sci. 2018, 14, 9–14. [Google Scholar]
- Putra, N.R.; Rizkiyah, D.N.; Zaini, A.S.; Yunus, M.A.C.; Machmudah, S.; binti Idham, Z.; Hazwan Ruslan, M.S. Effect of particle size on yield extract and antioxidant activity of peanut skin using modified supercritical carbon dioxide and soxhlet extraction. J. Food Process. Preserv. 2018, 42, 13689. [Google Scholar] [CrossRef]
- Siqungathi, Z.; Madzimbamuto, T.F.N.; Ojumu, T. V Multi—Product Fractionation of Roasted Peanuts using scCO2: An Evaluation of Process Alternatives. In Proceedings of the 17th JOHANNESBURG Int’l Conference on Science, Engineering, Technology & Waste Management (SETWM-19), Johannesburg, South Africa, 18–19 November 2019; pp. 18–20. [Google Scholar]
- Carrín, M.E.; Crapiste, G.H. Mathematical modeling of vegetable oil-solvent extraction in a multistage horizontal extractor. J. Food Eng. 2008, 85, 418–425. [Google Scholar] [CrossRef]
- Li, P.; Gasmalla, M.A.A.; Zhang, W.; Liu, J.; Bing, R.; Yang, R. Effects of roasting temperatures and grinding type on the yields of oil and protein obtained by aqueous extraction processing. J. Food Eng. 2016, 173, 15–24. [Google Scholar] [CrossRef]
- Lopes, G.d.S.; de Araujo, P.C.C.; da Silva, M.J.; Paim, L.L.; de Oliveira, K.R.; Valarini Junior, O.; Favareto, R.; Parizi, M.P.S.; Ferreira-Pinto, L. Kinetic study of peanut seed oil extraction with supercritical CO2. Res. Soc. Dev. 2022, 11, e15511427098. [Google Scholar] [CrossRef]
- Putra, N.R.; Idham, Z.B.; Machmudah, S.; bin Ruslan, M.S.H.; Che Yunus, M.A. Extraction of peanut skin oil by modified supercritical carbon dioxide: Empirical modelling and optimization. Sep. Sci. Technol. 2018, 53, 2695–2703. [Google Scholar] [CrossRef]
- Rosenthal, A.; Pyle, D.L.; Niranjan, K. Aqueous and enzymatic processes for edible oil extraction. Enzym. Microb. Technol. 1996, 19, 402–420. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Aziz, A.H.A.; Idham, Z.; Jumakir, J.; Waluyo, W.; Yunus, M.A.C. Application of drying model to determine extraction behaviours on peanut skin oil recovery by supercritical carbon dioxide-ethanol. Malays. J. Fundam. Appl. Sci. 2021, 17, 114–127. [Google Scholar] [CrossRef]
- Liu, J.J.; Gasmalla, M.A.A.; Li, P.; Yang, R. Enzyme-assisted extraction processing from oilseeds: Principle, processing and application. Innov. Food Sci. Emerg. Technol. 2016, 35, 184–193. [Google Scholar] [CrossRef]
- Nguyen, T.; Do, L.; Sabatini, D.A. Biodiesel production via peanut oil extraction using diesel-based reverse-micellar microemulsions. Fuel 2010, 89, 2285–2291. [Google Scholar] [CrossRef]
- Zhang, S.B.; Lu, Q.Y.; Yang, H.; Li, Y.; Wang, S. Aqueous enzymatic extraction of oil and protein hydrolysates from roasted peanut seeds. JAOCS J. Am. Oil Chem. Soc. 2011, 88, 727–732. [Google Scholar] [CrossRef]
- Virot, M.; Tomao, V.; Ginies, C.; Visinoni, F.; Chemat, F. Microwave-integrated extraction of total fats and oils. J. Chromatogr. A 2008, 1196–1197, 57–64. [Google Scholar] [CrossRef]
- Liu, C.; Chen, F.S.; Xia, Y.M. Composition and structural characterization of peanut crude oil bodies extracted by aqueous enzymatic method. J. Food Compos. Anal. 2022, 105, 104238. [Google Scholar] [CrossRef]
- Mushtaq, A.; Roobab, U.; Denoya, G.I.; Inam-Ur-Raheem, M.; Gullón, B.; Lorenzo, J.M.; Barba, F.J.; Zeng, X.A.; Wali, A.; Aadil, R.M. Advances in green processing of seed oils using ultrasound-assisted extraction: A review. J. Food Process. Preserv. 2020, 44, 14740. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Singh, V.K.; Garg, M.K. Novel oil extraction technologies: Process conditions, quality parameters, and optimization. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3–20. [Google Scholar] [CrossRef]
- Niu, R.; Chen, F.; Liu, C.; Duan, X. Composition and rheological properties of peanut oil bodies from aqueous enzymatic extraction. J. Oleo Sci. 2021, 70, 375–383. [Google Scholar] [CrossRef]
- Liu, C.; Hao, L.; Chen, F.; Yang, C. Study on extraction of peanut protein and oil bodies by aqueous enzymatic extraction and characterization of protein. J. Chem. 2020, 2020, 5148967. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lamsal, B.P. Ultrasound-assisted extraction and modification of plant-based proteins: Impact on physicochemical, functional, and nutritional properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1457–1480. [Google Scholar] [CrossRef] [PubMed]
- Megahed, M.G. Microwave roasting of peanuts: Effects on oil characteristics and composition. Nahr. Food 2001, 45, 255–257. [Google Scholar] [CrossRef]
- Guo, C.; Xie, Y.J.; Zhu, M.T.; Xiong, Q.; Chen, Y.; Yu, Q.; Xie, J.H. Influence of different cooking methods on the nutritional and potentially harmful components of peanuts. Food Chem. 2020, 316, 126269. [Google Scholar] [CrossRef]
- Savoire, R.; Lanoisellé, J.L.; Vorobiev, E. Mechanical Continuous Oil Expression from Oilseeds: A Review. Food Bioprocess Technol. 2013, 6, 1–16. [Google Scholar] [CrossRef]
- El Idrissi, Z.L.; El Moudden, H.; Mghazli, N.; Bouyahya, A.; Guezzane, C.E.; Alshahrani, M.M.; Al Awadh, A.A.; Goh, K.W.; Ming, L.C.; Harhar, H.; et al. Effects of Extraction Methods on the Bioactivities and Nutritional Value of Virginia and Valencia-Type Peanut Oil. Molecules 2022, 27, 7709. [Google Scholar] [CrossRef]
- James, R.; Baxley, E. Partially Defatted Nut Coating and Reconstituting Process. United. States Patent US 3,740,236, 19 June 1973. [Google Scholar]
- Peter, I.; Gannis, M.; Bosco, P.M.; Junction, H. Process for Treating Partially Defatted Nuts. U.S. Patent US 4,049,833, 20 September 1977. [Google Scholar]
- Holloway, J.; Wilkins, H. Low-fat nuts with improved natural flavor. Am. Pat 1982, 4, 329. [Google Scholar]
- Gannis, P.; Wilkins, H. Method of Producing Flavor Infused Partially Defatted Nuts and Product. U.S. Patent US 5,002,802, 26 March 1991. [Google Scholar]
- Chand, A.; Passey, N.D.P. Process for Preparing Low-Calorie Nuts. U.S. Patent US 5,290,578, 1 March 1994. [Google Scholar]
- Zook, D.; Ruth, A.; Wheeler, M.E.; Otterburn, J.F. Product and Process of Making Low Calorie Nuts. U.S. Patent US 5,240,726, 31 August 1993. [Google Scholar]
- Wong, V.Y.; Sackenheim, R.J. Process of Making Low Fat Nuts. U.S. Patent US 5,164,217, 17 November 1992. [Google Scholar]
- Boyce, I.R.C.; Jones, M.C.; Festa, E.J. Method of Producing a Reduced Fat Peanut Butter without Non-Peanut Supplements and Product. U.S. Patent US 5,591,477, 7 January 1997. [Google Scholar]
- Allaf, K.; Maache-Rezzoug, Z.; Rezzoug, S.; Habba, A.; Louka, E.D.; Abraham, G.; Louka, N. Method for Treating Various Products and Installation. U.S. Patent US 6,551,644, 22 April 2003. [Google Scholar]
- Ōgaki, N.I.; Itsuki Sagawa, Y.O. Production of Soy Sauce Using Defatted Peanut. JP20417398A, 16 August 1998. [Google Scholar]
- York-Leung Wong, V.; Waimin Sui, S.; Sackenheim, R.J. Low Fat Nutspread Composition with High Protein and Fiber. U.S. Patent US 6,706,311, 16, March 2004. [Google Scholar]
- Xavier, L. Low-Calorie, Low-Fat Snack Nuts. U.S. Patent US 8,715,767, 6 May 2014. [Google Scholar]
- Wang, Q.; Jiao, B.; Chen, Y.; Hu, H. Chewing-Resistant Semi-Defatted Leisure Peanut and Preparation Method. CN 113383932 A, 25 June 2021. [Google Scholar]
- Wang, Q.; Jiao, B.; Chen, Y.; Hu, H. Semi-Defatted Crispy Peanut with High Whole Grain Rate and Preparation Method. CN113995110A, 21 October 2021. [Google Scholar]
- Wu, H.; Wang, Q.; Ma, T.; Ren, J. Comparative studies on the functional properties of various protein concentrate preparations of peanut protein. Food Res. Int. 2009, 42, 343–348. [Google Scholar] [CrossRef]
- Boukid, F. Peanut protein—An underutilised by-product with great potential: A review. Int. J. Food Sci. Technol. 2022, 57, 5585–5591. [Google Scholar] [CrossRef]
- Traoret, C.J.; Lokko, P.; Cruz, A.C.R.F.; Oliveira, C.G.; Costa, N.M.B.; Bressan, J.; Alfenas, R.C.G.; Mattes, R.D. Peanut digestion and energy balance. Int. J. Obes. 2008, 32, 322–328. [Google Scholar] [CrossRef]
- Yildirim, Ö.; Acar, Ü. Defatted peanut meal (Arachis hypogea) as a complementary protein source in diets for Mozambique tilapia (Oreochromis mossambicus) fry and effect on fatty acid composition. Pak. J. Zool. 2016, 48, 377–382. [Google Scholar]
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as functional food: A review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef]
- Gbadamosi, S.O.; Abiose, S.H.; Aluko, R.E. Amino acid profile, protein digestibility, thermal and functional properties of Conophor nut (Tetracarpidium conophorum) defatted flour, protein concentrate and isolates. Int. J. Food Sci. Technol. 2012, 47, 731–739. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Yu, L.; Wu, K.; Zhao, M. Emulsification performance and interfacial properties of enzymically hydrolyzed peanut protein isolate pretreated by extrusion cooking. Food Hydrocoll. 2018, 77, 607–616. [Google Scholar] [CrossRef]
- Jain, A.; Prakash, M.; Radha, C. Extraction and evaluation of functional properties of groundnut protein concentrate. J. Food Sci. Technol. 2015, 52, 6655–6662. [Google Scholar] [CrossRef]
- Feng, H.; Jin, H.; Gao, Y.; Yan, S.; Zhang, Y.; Zhao, Q.; Xu, J. Effects of freeze-thaw cycles on the structure and emulsifying properties of peanut protein isolates. Food Chem. 2020, 330, 127215. [Google Scholar] [CrossRef]
- Ma, T.; Zhu, H.; Wang, J.; Wang, Q.; Yu, L.L.; Sun, B. Influence of extraction and solubilizing treatments on the molecular structure and functional properties of peanut protein. LWT 2017, 79, 197–204. [Google Scholar] [CrossRef]
- Haidar, E.; Lakkis, J.; Karam, M.; Koubaa, M.; Louka, N.; Debs, E. Peanut Allergenicity: An Insight into Its Mitigation Using Thermomechanical Processing. Foods 2023, 12, 1253. [Google Scholar] [CrossRef]
| Methods/Year/Reference | Target Product(s) | Product(s) Description | Nutritional Value | Oil Recovery (%) | Pre-Treatment | Post-Treatment | Parameters |
|---|---|---|---|---|---|---|---|
| MEPSI (2014) [1] | Partially defatted peanuts and Peanut oil. | Expansion, preserving structural integrity, and organoleptic properties | High protein content in the defatted peanut | 50 (Optimal conditions) up to 80% |
| IVDV texturization: P = 0.9 MPa, 10 s | Pressing optimal conditions:
|
| MEPSI optimized by RSM (2016) [3] | Partially defatted peanuts and Peanut oil. | Expansion, recovering original shape, and organoleptic properties | High-protein, crunchy snacks with reduced fat | 70–80% |
| Reconstitution and roasting:
| Pressing optimal conditions:
|
| MEPSI optimized by RSM (2016) [2] | Partially defatted peanuts and Peanut oil. | Expansion, recovering original shape, and organoleptic properties | High protein, low fat, and high fiber content in the defatted peanut | 70.6% |
| Reconstitution and roasting:
| Pressing optimal conditions:
|
| MEPSI optimized by RSM (2017) [8] | Partially defatted peanuts and Peanut oil. | Full shape recovery of peanuts, assuring better morphological, organoleptic, and rheological properties | High protein, low fat, and high fiber content in the defatted peanut | 56.26% |
|
| IVDV optimal conditions:
|
| MEPSI optimized by RSM (2018) [4] | Partially defatted peanuts and Peanut oil. | Full shape recovery of peanuts, assuring better morphological, organoleptic, and rheological properties | High protein, low fat, and high fiber content in the defatted peanut | 45.02 ± 0.4% |
|
| IVDV optimal conditions:
|
| MEPSI optimized by RSM (2021) [22] | Partially defatted peanuts and Peanut oil. | Full shape recovery of peanuts, assuring better morphological, organoleptic, and rheological properties | High protein, low fat, and high fiber content in the defatted peanut | 70.62% |
|
| IVDV optimal conditions:
|
| Hot and cold press (2020) [6] | Peanut Oil | Formation of PDPM | Low fat, High protein, and high fiber content in the PDPM | N/A | Roasting: 180 °C, 20 min (With or without removing the red skin). | N/A | Pressing optimal conditions:
|
| Hydraulic press (pre-treated with IR irradiation) (2020) [44] | Partially defatted peanuts and Peanut oil. | Full shape recovery of peanuts, assuring better morphological, organoleptic, and rheological properties | Rich in fiber and more than 30 essential nutrients. High concentrations of polyphenols and antioxidants in the defatted peanut | 45% |
| IVDV texturization. | Process optimal conditions:
|
| Extrusion optimized by RSM (2009) [15] | PDPF and Peanut oil. | PDPF was used to develop peanut-based Textured Meat Analogue | High protein content and cholesterol-free in the peanut-based TMA | N/A | N/A | N/A | Extrusion Optimal conditions:
|
| Screw pressing (2019) [17] | Peanut oil | The peanuts exhibit compromised integrity characteristics of oil were investigated. | Peanut oil is a rich source of bioactive components | -Roasted peanuts: 41.18–46.28%. -Non-roasted peanuts: 41.17% |
| Centrifugation of peanut oil: 12,000 rpm, 10 min. |
|
| MEPSI optimized by RSM (2016) [45] | Partially defatted peanuts and Peanut oil. | Full shape recovery of peanuts, assuring better morphological, organoleptic, and rheological properties | High protein, low fat, and high fiber content in the defatted peanut | 70% |
|
| Pressing optimal conditions:
|
| Dry, wet extrusion and Screw Pressing (2009) [16] | PDPF and Peanut oil. | Formation of PDPM | High protein, low fat, and high fiber content in the PDPM | 65.6% extruder only vs. 90.6% extrusion and screw pressing | Dehulling and separating of skins. | N/A | Extrusion optimal conditions: 136–138 °C, 30 s, feed rate = 142 kg/h. Pressing optimal conditions: T = 90 °C, 1 min discharge opening in the screw press: 4.49 mm. |
| Cold pressing (2018) [19] | PDPM and Peanut oil. | Formation of PDPM | High-quality oils are obtained suitable for direct consumption | 65% |
| N/A | Pressing experimental conditions:
|
| Cold pressing (2020) [21] | PDPM and Peanut oil. | Formation of PDPM | Low fat, High protein (>25%), and high fiber content in the PDPM | 30% |
| N/A | Pressing experimental conditions:
|
| Cold pressing (2017) [20] | PDPM and Peanut oil. | Formation of PDPM | High protein, with reduced fat in the PDPM. Oil is in line with national standards. | 39.8% |
| Filtering: Peanut oil. | Pressing optimal conditions:
|
| Screw Pressing (2020) [46] | PDPM and Peanut oil. | Formation of PDPM | Peanut oil contains oleic and linoleic acids (38–56%) and (16–38%), respectively, and is low in free fatty acids | 75.89% |
| N/A | Pressing optimal conditions:
|
| Hydraulic Pressing (2007) [47] | Peanut oil | Formation of PDPM | High protein, with reduced fat in the PDPM. | 33.36% | N/A | N/A | Pressing optimal conditions:
|
| Hydraulic Pressing (2014) [48] | Peanut oil | Formation of PDPM | High protein, with reduced fat in the PDPM. | 32.36% | Drying: 130 °C, 6 h | N/A | Pressing optimal conditions:
|
| Methods/Year/Reference | Target Product(s) | Product(s) Description | Nutritional Value | Oil Recovery (%) | Pre-Treatment | Post-Treatment | Parameters |
|---|---|---|---|---|---|---|---|
| Organic solvent extraction (2020) [6] | PDPM and Peanut oil | Formation of PDPM | High protein, low fat, and high fiber content with a medium amount of carbs, vitamins, and minerals in the PDPM | N/A | Deskinning of peanuts. | Removal of Hexane using a rotary vacuum evaporator | Optimal conditions for oil extraction with Hexane by thermal cycles:
|
| SC-CO2 Extraction (1996) [59] | PDPM and Peanut oil | Formation of PDPM | N/A | 95% |
| N/A | Intermittently depressurized process optimal conditions:
|
| Soxhlet extraction (2017) [60] | Peanut Oil | Peanuts are completely defatted but not fit for consumption | N/A |
| N/A |
| Soxhlet optimal conditions:
|
| SC-CO2 Extraction and Soxhlet Extraction (2018) [57] | Peanut skin oil | Peanut skin powder | Peanut skins are rich in antioxidants such as procyanidin, catechin, and epicatechin. | SC-CO2: 15.47% oil extract from the total product Soxhlet: 36.282% → (SC-CO2: ~42.63% Soxhlet: 100%) |
| N/A | Soxhlet Extraction optimal conditions:
|
| SC-CO2 Extraction (2022) [61] | Peanut Oil | Integrity of peanuts is compromised | N/A | Increasing temperature of extraction: 50 °C→60 °C, oil recovery increase on a mass basis by 12.2% | N/A | CO2 compression and recycling | SFE optimal conditions:
|
| SC-CO2 Extraction with co-solvent ethanol (2018) [62] | Peanut skin oil | Peanut skin powder | Peanut skins are rich in antioxidants such as procyanidin, catechin, and epicatechin. | 14.95% Total product mass oil extract | Pulverize thoroughly | N/A | SFE optimal conditions:
|
| SC-CO2 Extraction and Soxhlet extraction (2018) [63] | Peanut skin oil | Peanut skin powder | Peanut skins are rich in antioxidants such as procyanidin, catechin, and epicatechin. | SC-CO2:15.53% Total product mass oil extract Soxhlet: 36.28% → (SC-CO2: ~42.8% Soxhlet: 100%) |
| N/A | SFE optimal conditions:
|
| Organic solvent Extraction (2019) [17] | Peanut oil | Partially defatted peanuts (not fit for consumption) and Peanut oil | Peanuts are a rich source of bioactive components | Roasted peanuts: 47.77–55.35% Non-roasted peanuts: 47.75% |
| N/A | Experimental conditions for oil extraction with Hexane:
|
| SC-CO2 Extraction (2019) [64] | Peanut oil | The integrity of peanuts is compromised | Peanut oil is an important source of edible oils, aroma compounds, and fatty acids, particularly oleic (18:1) and linoleic (18:2) acid | 15.50% Total product mass oil extract | Drying: 80 °C, 24 h | N/A | SFE experimental conditions:
|
| Organic solvent extraction (2008) [65] | Peanut Oil | Partially defatted peanuts (not fit for consumption) and Peanut oil | N/A | 85% | N/A | N/A | Oil industrial extractor with the following characteristics:
|
| AEP (2016) [66] | Peanut oil | Peanut Paste extracts: peanut oil, fiber precipitate, residual cream, and skim | N/A | 92.20% of free oil |
| N/A | AEP experimental conditions:
|
| SC-CO2 Extraction vs. Soxhlet extraction (2022) [67] | Peanut oil | The integrity of peanuts is compromised |
|
|
| N/A | SFE of peanut oil:
|
| SC-CO2 Extraction (2018) [68] | Peanut skin oil | Peanut skin powder | Peanut skin is rich in antioxidants and bioactive compounds | 0.83 g extract of 5 g peanuts skin (16.6% Total product mass oil extract) |
| N/A | SFE optimal conditions:
|
| AEP (1996) [69] | Peanut oil | Formation of either PPC or PPI in addition to peanut oil | N/A | 89% when pH = 4 86% when pH = 7 | Dry grinding of peanuts. | N/A | AEP experimental conditions:
|
| Organic solvent extraction (Alternative solvents to hexane) (1983) [26] | PDPM and Peanut oil | Formation of PDPM | N/A | N/A |
| N/A | N/A |
| SC-CO2 Extraction with co-solvent ethanol (2021) [70] | Peanut skin oil | Peanut skin powder | Peanut skins are rich in antioxidants such as procyanidin, catechin, and epicatechin. | 14–15% |
| N/A | SFE experimental conditions:
|
| Methods/Year/Reference | Target Product(s) | Product(s) Description | Nutritional Value | Oil Recovery (%) | Pre-Treatment | Post-Treatment | Parameters |
|---|---|---|---|---|---|---|---|
| Aqueous and mechanical extraction (2020) [30] | Peanut oil and PDPM. | Formation of PDPM and clarified-free oils | High protein content and low residual oil in the PDPM | 96.1 ± 0.2% |
|
| Combined experimental conditions:
|
| AEP combined with membrane separation (2020) [31] | Peanut oil, proteins, and insoluble fiber-rich solid residual fraction | The peanuts exhibit compromised integrity | N/A | AEP: 96.51 ± 1.14%. UF processing: 95.30 ± 0.78% |
|
| AEP experimental conditions:
|
| EAAE (2002) [27] | Peanut oil | The peanuts exhibit compromised integrity | Peanut seeds contain 27–29% protein and 40–50% oil | 86–92% |
| N/A | Extraction optimal conditions:
|
| Diesel-based reverse-micellar microemulsion extraction (2010) [72] | Peanut oil/diesel blend (biodiesel fuel) | The peanuts exhibit compromised integrity | N/A | 91.6 ± 2.5% | N/A |
| Extraction optimal conditions:
|
| Ultrasound-assisted Enzymatic Extraction (2018) [32] | Peanut oil and PDPM. | Formation of PDPM | Peanuts are a source of protein and oil. Peanut oil is rich in mono- and poly-unsaturated fatty acids. | An increase in oil yield by 30.61%, and 173.77% compared to n-hexane solvent extraction and AEP |
|
| Extraction optimal conditions:
|
| EAAE (2011) [73] | Peanut oil and PDPM. | Formation of PDPM | Peanut seeds contain 25–29% protein and 40–50% oil | 86–90% |
| Demulsification:
| Extraction optimal conditions:
|
| EAAE (2010) [28] | Peanut oil and PDPM. | Formation of PDPM | -Peanuts: high-quality oil (45–55%) and protein (24–36%). -Peanut oil: glyceride mixture of about 80% unsaturated fatty acids and 20% saturated fatty acids. | 91.98% |
| N/A | Extraction optimal conditions:
|
| Ultrasonic-assisted aqueous enzymatic extraction (2011) [33] | Peanut oil | Peanut Paste extracts: peanut oil, residual cream, and skim | Peanut oil mix: 40–50% oil and 27–29% protein, with high monounsaturated content. | 88% |
| N/A | Extraction optimal conditions:
|
| EAAE (2008) [29] | Peanut oil | Peanut Paste extracts: peanut oil, residual meal | Peanut seeds: 24–28% protein and 45–52% oil | 91.7 ± 1.3% |
|
| Extraction optimal conditions :
|
| Microwave-integrated Soxhlet (MIS) (2008) [74] | Peanut oil | The peanuts exhibit compromised integrity | The nine compounds: Palmitic (C16:0), palmitoleic (C16:1), margaroleic (C17:1), stearic (C18:0), oleic (C18:1), linoleic (C18:2), linolenic (C18:3), arachidic (C20:0), and gadoleic (C20:1) acids (98% of the total composition of identified fatty acids in the extracted oils). | 46.1% in 32 min. |
| N/A | MIS experimental conditions:
|
| EAAE (2022) [75] | POB | Peanut Paste extracts: peanut oil, residual meal | Peanuts: rich in protein (24.16%) and oil (51.43%). Fatty acids in crude Oil bodies are oleic acid (40.70%) and linoleic acid (35.01%). | N/A |
| N/A | Extraction experimental conditions:
|
| Ultrasound-assisted Soxhlet extraction (2020) [76] | Peanut oil | The peanuts exhibit compromised integrity | Peanut oils: rich in protein, monounsaturated, and polyunsaturated fatty acids | 51.50%, 10 min | N/A | N/A | Extraction experimental conditions:
|
| Short-wave IR radiation aqueous enzymatic extraction (2013) [34] | Peanut oil | The peanuts exhibit compromised integrity | Peanuts: 44–56% lipids, 22–30% protein, 16–25% carbohydrates, and a low percentage of minerals and vitamins. Oleic acid and linoleic acid: 80% of the total fatty acids in peanut oil. | 83.75 ± 2.90% |
| N/A | Extraction experimental conditions:
|
| EAAE (2020) [77] | Peanut oil | The peanuts exhibit compromised integrity | Peanuts lipid molecules integrate with the protein molecules and are surrounded by a cell wall containing cellulose, hemicelluloses, lignin, and pectin | 91.98% | N/A | N/A | Extraction experimental conditions:
|
| EAAE (1996) [69] | Peanut oil | The peanuts exhibit compromised integrity | N/A | 74–78% | Dry grinding. | N/A | Extraction experimental conditions:
|
| Salt-Assisted (CaCl2) Microwave Aqueous Enzymatic Extraction (2020) [35] | Peanut oil | Peanut Paste extracts: peanut oil, residual cream, and skim | N/A | 92.3%, 2 min | Grinding to paste | Demulsification using microwave radiation or by freezing-thawing and heating treatment | Extraction optimal conditions:
|
| EAAE (2021) [78] | POB | The peanuts exhibit compromised integrity | POBs are rich in essential amino acids, unsaturated fatty acids, vitamin E, and phytosterols. The oil bodies contained three inherent proteins (oleosin, caleosin, and steroleosin) along with two adsorbed foreign proteins (arachin and lipoxygenase). | 90.7% |
| N/A | Extraction optimal conditions:
|
| EAAE (2016) [71] | Peanut oil | The peanuts exhibit compromised integrity | N/A | 1st case: 92.2% 2nd case: 79.32% | Grinding + Enzyme exposure (alcalase 2.4 L). | N/A | Extraction optimal conditions:
|
| EAAE (2020) [79] | POB | The peanuts exhibit compromised integrity | Peanuts: 46.84% oil, 24.44% protein, 4.65% crude fiber, 4.63% water, 2.35% ash | 48.44% |
| N/A | Extraction optimal conditions:
|
| Methods | Advantages | Disadvantages | |
|---|---|---|---|
| Mechanical | Traditional Hydraulic Pressing | ||
| MEPSI |
|
| |
| Screw Pressing |
|
| |
| Cold Pressing |
| ||
| MEPSI combined with IVDV as a Post-treatment | |||
| Chemical Chemical | AEP | ||
| SC-CO2 extraction |
|
| |
| Soxhlet Extraction |
| ||
| Organic Solvent extraction |
|
| |
| Combined Combined | EAAE |
|
|
| Infrared pre-treatment | |||
| Diesel-based micellar emulsions |
| ||
| Ultrasound-assisted enzymatic extraction |
|
| |
| MIS |
| ||
| MAEE | - Care should be exercised as very high temperatures affect the nutritional and sensory characteristics of the final product [77] | ||
| Rapid Salt-Assisted Microwave Demulsification | - |
| Title/Year/Reference | Treatment Process | Target Products | Improvements/Innovations |
|---|---|---|---|
| Partially defatted nut coating and reconstituting process (1968) [85] |
| Partially Defatted nuts | Reconstitution of the nuts to their original shape and even 25% greater than their original shape. |
| Process for treating partially defatted nuts (1977) [86] | Heating the nuts with an aqueous solution containing V > 2% glycerol, 2 min, T > 65.55 °C | Partially defatted nuts |
|
| Low-Fat nuts with improved natural flavor (1982) [87] |
| Partially defatted nuts |
|
| Method of producing flavor-infused partially defatted nuts and products (1989) [88] |
| Partially defatted nuts |
|
| Process for preparing low-calorie nuts (1990) [89] |
| Partially defatted peanuts |
|
| Product and process of making low-calorie nuts (1992) [90] |
| Partially defatted nuts |
|
| Process of making low-fat nuts (1992) [91] |
| Partially defatted nuts |
|
| Method of producing a reduced-fat peanut butter without non-peanut supplements (1997) [92] |
| Low-fat peanut Butter |
|
| Method for treating various products and installations (1998) [93] |
| Various products |
|
| Production of soy sauce using defatted peanuts (2000) [94] |
| Soy Sauce |
|
| Low-fat nut spread composition with high protein and fiber (2004) [95] |
| Low-fat nut spread |
|
| The treatment process for biological products MEPSI aims at modifying their lipid content and their texture. Settings and methods for the implementation of such a process (2014) [55] | N/A | Partially defatted food products |
|
| Seeds and nuts are defatted by pressing and reconstituted by methods preserving their appearance and organoleptic properties (2014) [56] |
| Partially defatted nuts |
|
| Low-calories, low-fat snack nuts (2014) [96] |
| Partially defatted peanuts |
|
| Chewing-resistant semi-defatted leisure peanut and preparation method (2021) [97] |
| Partially defatted leisure peanuts |
|
| Semi-defatted crispy peanuts with a high whole grain rate and preparation method (2022) [98] |
| Partially defatted crispy peanut |
|
| Target Product(s) | Nutritional Value | Parameters | Reference |
|---|---|---|---|
| PPI and PDPM |
|
| [80] |
| PPC |
|
| [51] |
| PPC | PPC has more than 70 g protein/100 g product |
| [99] |
| PPI and PPC |
|
| [100] |
| Peanut, peanut oil, peanut butter, peanut flour | Peanuts: ≈50% fat and 45% carbohydrate and protein. Protein (% of total energy):
|
| [101] |
| PDPM | Fish diets:
|
| [102] |
| PPI and PPC |
|
| [103] |
| PDPM |
|
| [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahfoud, F.; Assaf, J.C.; Elias, R.; Debs, E.; Louka, N. Defatting and Defatted Peanuts: A Critical Review on Methods of Oil Extraction and Consideration of Solid Matrix as a By-Product or Intended Target. Processes 2023, 11, 2512. https://doi.org/10.3390/pr11082512
Mahfoud F, Assaf JC, Elias R, Debs E, Louka N. Defatting and Defatted Peanuts: A Critical Review on Methods of Oil Extraction and Consideration of Solid Matrix as a By-Product or Intended Target. Processes. 2023; 11(8):2512. https://doi.org/10.3390/pr11082512
Chicago/Turabian StyleMahfoud, Freddy, Jean Claude Assaf, Rudolph Elias, Espérance Debs, and Nicolas Louka. 2023. "Defatting and Defatted Peanuts: A Critical Review on Methods of Oil Extraction and Consideration of Solid Matrix as a By-Product or Intended Target" Processes 11, no. 8: 2512. https://doi.org/10.3390/pr11082512
APA StyleMahfoud, F., Assaf, J. C., Elias, R., Debs, E., & Louka, N. (2023). Defatting and Defatted Peanuts: A Critical Review on Methods of Oil Extraction and Consideration of Solid Matrix as a By-Product or Intended Target. Processes, 11(8), 2512. https://doi.org/10.3390/pr11082512