An Experimental Investigation of Hydrogen Production through Biomass Electrolysis
Abstract
:1. Introduction
2. Experiment
2.1. Materials
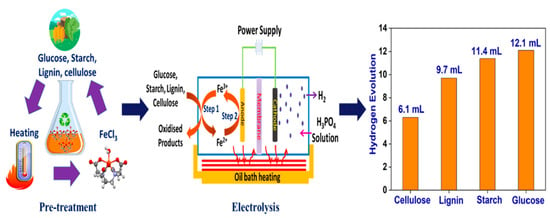
2.2. Selection of Feedstocks
2.3. Chemical Pre-Treatment of Biomass Components
2.4. Hydrogen Generation through Biomass Components via Electrolysis
3. Results and Discussion
3.1. Analysis of the Electrochemical Properties of FeCl3-Mediated Biomass Components during Electrolysis
3.2. Effect of Electrolysis Operating Temperature on the Electrochemical Conductivity during FeCl3-Mediated Biomass Electrolysis
3.3. Hydrogen Production via PEMEC following FeCl3 Pretreatment
4. Conclusions
- Replacing the noble metal catalyst at the anode with the low-cost Lewis acid as a liquid catalyst in the H-PEMEC for the catalytic reaction demonstrates the low-cost potential of the biomass electrolysis process.
- The use of the non-noble metal Fe+3/Fe+2 redox couple or ion pair for the conversion of biomass components towards sustainable H2 through the H-PEMEC shows the feasibility of the decomposition of biomass and hydrogen production at low-voltage and low-temperature conditions, suggesting an advantage of H2 production from biomass electrolysis compared to high-temperature processes like gasification and pyrolysis.
- The blank and char experiments confirm that there is no hydrogen produced when the biomass feedstock itself contains little to no hydrogen content. Therefore, when choosing a feedstock for biomass electrolysis, one of important factors should be the hydrogen content of the feedstock.
- The maximum H2 yields with the biomass electrolysis process vary from 6.3 to 12.1 mL when using cellulose, lignin, starch and glucose, although they all have similar H2 contents. This indicates that the feedstock structure is another factor influencing H2 generating rates.
- The power-to-H2 yield ratio in biomass electrolysis at ambient conditions for selected biomass components ranges from 30.99 to 33.83 kWh/kg of H2. In comparison with conventional water electrolysis (55–58 kWh/kg), the biomass electrolysis process is more energy-efficient for hydrogen production.
- CO2 emissions are also investigated during the FeCl3-mediated biomass electrolysis process for estimating the conversion of biomass feedstocks during the process. The results indicate that CO2 emissions during the process are low because the maximum carbon consumption rate is around 13%. Therefore, the carbon content in the residues is still more than 87% that can potentially be used for energy purposes.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, A.K.; Dunlop, P.S.; Bhattacharya, G.; Mokim, M.; Hewitt, N.J.; Huang, Y.; Gogulancea, V.; Zhang, K.; Brandoni, C. Comparative performance of sustainable anode materials in microbial fuel cells (MFCs) for electricity generation from wastewater. Results Eng. 2023, 20, 101385. [Google Scholar] [CrossRef]
- Hossen, M.A.; Solayman, H.; Leong, K.H.; Sim, L.C.; Nurashikin, Y.; Abd Aziz, A.; Wu, L.; Monir, M.U. Recent progress in TiO2-Based photocatalysts for conversion of CO2 to hydrocarbon fuels: A systematic review. Results Eng. 2022, 16, 100795. [Google Scholar] [CrossRef]
- World Energy Outlook. Available online: https://www.iea.org/reports/world-energy-outlook-2021 (accessed on 10 October 2023).
- Afrouzi, H.N.; Ahmed, J.; Siddique, B.M.; Khairuddin, N.; Hassan, A. A comprehensive review on carbon footprint of regular diet and ways to improving lowered emissions. Results Eng. 2023, 18, 101054. [Google Scholar] [CrossRef]
- Wood, G.; Baker, K. The Palgrave Handbook of Managing Fossil Fuels and Energy Transitions; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Klemeš, J.J.; Varbanov, P.S.; Ocłoń, P.; Chin, H.H. Towards efficient and clean process integration: Utilisation of renewable resources and energy-saving technologies. Energies 2019, 12, 4092. [Google Scholar] [CrossRef]
- Kaydouh, M.-N.; El Hassan, N. Thermodynamic simulation of the co-gasification of biomass and plastic waste for hydrogen-rich syngas production. Results Eng. 2022, 16, 100771. [Google Scholar] [CrossRef]
- Acharya, S.; Gupta, D.S.; Kishore, N. In-situ catalytic hydro-liquefaction of Delonix regia lignocellulosic biomass waste in hydrogen-donor solvent. Results Eng. 2022, 16, 100734. [Google Scholar] [CrossRef]
- Jaffar, M.M.; Nahil, M.A.; Williams, P.T. Pyrolysis-catalytic hydrogenation of cellulose-hemicellulose-lignin and biomass agricultural wastes for synthetic natural gas production. J. Anal. Appl. Pyrolysis 2020, 145, 104753. [Google Scholar] [CrossRef]
- Nunes, L.J. Biomass gasification as an industrial process with effective proof-of-concept: A comprehensive review on technologies, processes and future developments. Results Eng. 2022, 14, 100408. [Google Scholar] [CrossRef]
- Aravindan, M.; Kumar, P. Hydrogen towards sustainable transition: A review of production, economic, environmental impact and scaling factors. Results Eng. 2023, 20, 101456. [Google Scholar]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield–a review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Reyes-Bozo, L.; Salazar, J.L.; Valdés-González, H.; Sandoval-Yáñez, C.; Vivanco-Soffia, M.E.; Bilartello, L.; Poblete, V.; Soto, A.; Urrea, M.J. Viability analysis for use of methane obtained from green hydrogen as a reducing agent in copper smelters. Results Eng. 2021, 12, 100286. [Google Scholar] [CrossRef]
- Liu, W.; Liu, C.; Gogoi, P.; Deng, Y. Overview of biomass conversion to electricity and hydrogen and recent developments in low-temperature electrochemical approaches. Engineering 2020, 6, 1351–1363. [Google Scholar] [CrossRef]
- Kumar, G.; Dharmaraja, J.; Arvindnarayan, S.; Shoban, S.; Bakonyi, P.; Saratale, G.D.; Nemestóthy, N.; Bélafi-Bakó, K.; Yoon, J.J.; Kim, S.H. A comprehensive review on thermochemical, biological, biochemical and hybrid conversion methods of bio-derived lignocellulosic molecules into renewable fuels. Fuel 2019, 251, 352–367. [Google Scholar] [CrossRef]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen production from biomasses and wastes: A technological review. Int. J. Hydrogen Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Li, M.; Wang, T.; Zhao, M.; Wang, Y. Research on hydrogen production and degradation of corn straw by circular electrolysis with polyoxometalate (POM) catalyst. Int. J. Hydrogen Energy 2022, 47, 15357–15369. [Google Scholar] [CrossRef]
- Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-hydrogen: A review of main routes production, processes evaluation and techno-economical assessment. Biomass Bioenergy 2021, 144, 105920. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Pirone, R.; Bensaid, S. Aqueous phase reforming process for the valorization of wastewater streams: Application to different industrial scenarios. Catal. Today 2022, 387, 224–236. [Google Scholar] [CrossRef]
- Pipitone, G.; Zoppi, G.; Frattini, A.; Bocchini, S.; Pirone, R.; Bensaid, S. Aqueous phase reforming of sugar-based biorefinery streams: From the simplicity of model compounds to the complexity of real feeds. Catal. Today 2020, 345, 267–279. [Google Scholar] [CrossRef]
- Godina, L.I.; Heeres, H.; Garcia, S.; Bennett, S.; Poulston, S.; Murzin, D.Y. Hydrogen production from sucrose via aqueous-phase reforming. Int. J. Hydrogen Energy 2019, 44, 14605–14623. [Google Scholar] [CrossRef]
- Luo, H.; Barrio, J.; Sunny, N.; Li, A.; Steier, L.; Shah, N.; Stephens, I.E.; Titirici, M.M. Progress and perspectives in photo-and electrochemical-oxidation of biomass for sustainable chemicals and hydrogen production. Adv. Energy Mater. 2021, 11, 2101180. [Google Scholar] [CrossRef]
- Dolle, C.; Neha, N.; Coutanceau, C. Electrochemical hydrogen production from biomass. Curr. Opin. Electrochem. 2022, 31, 100841. [Google Scholar] [CrossRef]
- Du, X.; Liu, W.; Zhang, Z.; Mulyadi, A.; Brittain, A.; Gong, J.; Deng, Y. Low-energy catalytic electrolysis for simultaneous hydrogen evolution and lignin depolymerization. ChemSusChem 2017, 10, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Sahu, O. Sustainable and clean treatment of industrial wastewater with microbial fuel cell. Results Eng. 2019, 4, 100053. [Google Scholar] [CrossRef]
- Gautam, R.; Ress, N.V.; Wilckens, R.S.; Ghosh, U.K. Hydrogen production in microbial electrolysis cell and reactor digestate valorization for biochar–a noble attempt towards circular economy. Int. J. Hydrogen Energy 2023, 52, 649–668. [Google Scholar] [CrossRef]
- Lu, L.; Xing, D.; Xie, T.; Ren, N.; Logan, B.E. Hydrogen production from proteins via electrohydrogenesis in microbial electrolysis cells. Biosens. Bioelectron. 2010, 25, 2690–2695. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Liu, Z.; He, Y.; Zhang, Y.; Lu, J.; Zhu, Z.; Si, B.; Zhang, C.; Xing, X.-H. Microbial electrolysis cell to treat hydrothermal liquefied wastewater from cornstalk and recover hydrogen: Degradation of organic compounds and characterization of microbial community. Int. J. Hydrogen Energy 2016, 41, 4132–4142. [Google Scholar] [CrossRef]
- Kadier, A.; Al-Shorgani, N.K.; Jadhav, D.A.; Sonawane, J.M.; Mathuriya, A.S.; Kalil, M.S.; Hasan, H.A.; Alabbosh, K.F.S. Microbial Electrolysis Cell (MEC) An Innovative Waste to Bioenergy and Value-Added By-product Technology. In Bioelectrosynthesis: Principles and Technologies for Value-Added Products; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2020; pp. 95–128. [Google Scholar]
- Islam, A.K.; Dunlop, P.S.; Hewitt, N.J.; Lenihan, R.; Brandoni, C. Bio-hydrogen production from wastewater: A comparative study of low energy intensive production processes. Clean Technol. 2021, 3, 156–182. [Google Scholar] [CrossRef]
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis–A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar]
- Xie, Z.; Yu, S.; Ma, X.; Li, K.; Ding, L.; Wang, W.; Cullen, D.A.; Meyer, H.M., III; Yu, H.; Tong, J.; et al. MoS2 nanosheet integrated electrodes with engineered 1T-2H phases and defects for efficient hydrogen production in practical PEM electrolysis. Appl. Catal. B Environ. 2022, 313, 121458. [Google Scholar] [CrossRef]
- Ayers, K. High efficiency PEM water electrolysis: Enabled by advanced catalysts, membranes, and processes. Curr. Opin. Chem. Eng. 2021, 33, 100719. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Guenot, B.; Cretin, M.; Lamy, C. Electrochemical reforming of dimethoxymethane in a Proton Exchange Membrane Electrolysis Cell: A way to generate clean hydrogen for low temperature fuel cells. Int. J. Hydrogen Energy 2017, 42, 28128–28139. [Google Scholar] [CrossRef]
- Coutanceau, C.; Baranton, S. Electrochemical conversion of alcohols for hydrogen production: A short overview. WIREs Energy Environ. 2016, 5, 388–400. [Google Scholar] [CrossRef]
- Guenot, B.; Cretin, M.; Lamy, C. Clean hydrogen generation from the electrocatalytic oxidation of methanol inside a proton exchange membrane electrolysis cell (PEMEC): Effect of methanol concentration and working temperature. J. Appl. Electrochem. 2015, 45, 973–981. [Google Scholar] [CrossRef]
- Lamy, C.; Devadas, A.; Simoes, M.; Coutanceau, C. Clean hydrogen generation through the electrocatalytic oxidation of formic acid in a Proton Exchange Membrane Electrolysis Cell (PEMEC). Electrochim. Acta 2012, 60, 112–120. [Google Scholar] [CrossRef]
- Marshall, A.; Haverkamp, R. Production of hydrogen by the electrochemical reforming of glycerol–water solutions in a PEM electrolysis cell. Int. J. Hydrogen Energy 2008, 33, 4649–4654. [Google Scholar] [CrossRef]
- Serrano-Jiménez, J.; de la Osa, A.; Rodríguez-Gómez, A.; Sánchez, P.; Romero, A.; de Lucas-Consuegra, A. Electro-reforming of bioethanol produced by sugar fermentation on a Pt-Ni anodic catalyst supported on graphene nanoplatelets. J. Environ. Chem. Eng. 2023, 11, 109703. [Google Scholar] [CrossRef]
- Liu, W.; Cui, Y.; Du, X.; Zhang, Z.; Chao, Z.; Deng, Y. High efficiency hydrogen evolution from native biomass electrolysis. Energy Environ. Sci. 2016, 9, 467–472. [Google Scholar] [CrossRef]
- Liu, W.; Mu, W.; Liu, M.; Zhang, X.; Cai, H.; Deng, Y. Solar-induced direct biomass-to-electricity hybrid fuel cell using polyoxometalates as photocatalyst and charge carrier. Nat. Commun. 2014, 5, 3208. [Google Scholar] [CrossRef]
- Rosa, L.; Mazzotti, M. Potential for hydrogen production from sustainable biomass with carbon capture and storage. Renew. Sustain. Energy Rev. 2022, 157, 112123. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Wang, T.; Li, M.; Lu, X.; Li, B. Study on hydrogen generation and cornstalk degradation by redox coupling of non-noble metal Fe3+/Fe2+. Int. J. Hydrogen Energy 2021, 46, 27409–27421. [Google Scholar] [CrossRef]
- Li, J.; Zhou, W.; Huang, Y.; Gao, J. Lignin-assisted water electrolysis for energy-saving hydrogen production with Ti/PbO2 as the anode. Front. Energy Res. 2021, 9, 762346. [Google Scholar] [CrossRef]
- Yang, L.; Liu, W.; Zhang, Z.; Du, X.; Gong, J.; Dong, L.; Deng, Y. Hydrogen evolution from native biomass with Fe3+/Fe2+ redox couple catalyzed electrolysis. Electrochim. Acta 2017, 246, 1163–1173. [Google Scholar] [CrossRef]
- Svensson, S.E.; Bucuricova, L.; Ferreira, J.A.; Souza Filho, P.F.; Taherzadeh, M.J.; Zamani, A. Valorization of bread waste to a fiber- and protein-rich fungal biomass. Fermentation 2021, 7, 91. [Google Scholar] [CrossRef]
- Bridgeman, T.; Jones, J.; Shield, I.; Williams, P.T. Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 2008, 87, 844–856. [Google Scholar] [CrossRef]
- Ghanim, B.M.; Kwapinski, W.; Leahy, J.J. Hydrothermal carbonisation of poultry litter: Effects of initial pH on yields and chemical properties of hydrochars. Bioresour. Technol. 2017, 238, 78–85. [Google Scholar] [CrossRef]
- Phyllis2. Available online: https://phyllis.nl/ (accessed on 10 October 2023).
- Li, M.; Wang, T.; Chen, X.; Ma, X. Conversion study from lignocellulosic biomass and electric energy to H2 and chemicals. Int. J. Hydrogen Energy 2023, 48, 21004–21017. [Google Scholar] [CrossRef]
- Mao, L.; Zhang, L.; Gao, N.; Li, A. FeCl3 and acetic acid co-catalyzed hydrolysis of corncob for improving furfural production and lignin removal from residue. Bioresour. Technol. 2012, 123, 324–331. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, B.; Wang, Q.; Xu, G.; Li, N.; Liu, W.; Zhang, Z.C. Energy-efficient Electrochemical Hydrogen Production Combined with Biomass Oxidation Using Polyoxometalate and Metal Salts. ChemCatChem 2023, 15, e202300522. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, C.; Liu, W.; Du, X.; Cui, Y.; Gong, J.; Guo, H.; Deng, Y. Direct conversion of sewage sludge to electricity using polyoxomatelate catalyzed flow fuel cell. Energy 2017, 141, 1019–1026. [Google Scholar] [CrossRef]
- Gaspar, A.R.; Gamelas, J.A.; Evtuguin, D.V.; Neto, C.P. Alternatives for lignocellulosic pulp delignification using polyoxometalates and oxygen: A review. Green Chem. 2007, 9, 717–730. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; Wang, Y.; Wu, L.; Hu, Y. Experimental and modeling of conductivity for electrolyte solution systems. ACS Omega 2020, 5, 22465–22474. [Google Scholar] [CrossRef] [PubMed]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Kozliak, E.I.; Kubátová, A.; Artemyeva, A.A.; Nagel, E.; Zhang, C.; Rajappagowda, R.B.; Smirnova, A.L. Thermal liquefaction of lignin to aromatics: Efficiency, selectivity, and product analysis. ACS Sustain. Chem. Eng. 2016, 4, 5106–5122. [Google Scholar] [CrossRef]
- Serrano-Jiménez, J.; de la Osa, A.; Rodríguez-Gómez, A.; Sánchez, P.; Romero, A.; de Lucas-Consuegra, A. Graphene-like materials as an alternative to carbon Vulcan support for the electrochemical reforming of ethanol: Towards a complete optimization of the anodic catalyst. J. Electroanal. Chem. 2022, 921, 116680. [Google Scholar] [CrossRef]
- Caravaca, A.; de Lucas-Consuegra, A.; Calcerrada, A.; Lobato, J.; Valverde, J.; Dorado, F. From biomass to pure hydrogen: Electrochemical reforming of bio-ethanol in a PEM electrolyser. Appl. Catal. B Environ. 2013, 134, 302–309. [Google Scholar] [CrossRef]
- Khalid, M.; De, B.S.; Singh, A.; Shahgaldi, S. Lignin Electrolysis at Room Temperature on Nickel Foam for Hydrogen Generation: Performance Evaluation and Effect of Flow Rate. Catalysts 2022, 12, 1646. [Google Scholar] [CrossRef]
- Caravaca, A.; Garcia-Lorefice, W.E.; Gil, S.; de Lucas-Consuegra, A.; Vernoux, P. Towards a sustainable technology for H2 production: Direct lignin electrolysis in a continuous-flow Polymer Electrolyte Membrane reactor. Electrochem. Commun. 2019, 100, 43–47. [Google Scholar] [CrossRef]
- Li, W.; Tian, H.; Ma, L.; Wang, Y.; Liu, X.; Gao, X. Low-temperature water electrolysis: Fundamentals, progress, and new strategies. Mater. Adv. 2022, 3, 5598–5644. [Google Scholar] [CrossRef]
- Jiang, Z.; Ding, W.; Xu, S.; Remón, J.; Shi, B.; Hu, C.; Clark, J.H. A ‘Trojan horse strategy’for the development of a renewable leather tanning agent produced via an AlCl 3-catalyzed cellulose depolymerization. Green Chem. 2020, 22, 316–321. [Google Scholar] [CrossRef]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Ito, M.; Hori, T.; Teranishi, S.; Nagao, M.; Hibino, T. Intermediate-temperature electrolysis of energy grass Miscanthus sinensis for sustainable hydrogen production. Sci. Rep. 2018, 8, 16186. [Google Scholar] [CrossRef] [PubMed]
- Kakoulaki, G.; Kougias, I.; Taylor, N.; Dolci, F.; Moya, J.; Jäger-Waldau, A. Green hydrogen in Europe–A regional assessment: Substituting existing production with electrolysis powered by renewables. Energy Convers. Manag. 2021, 228, 113649. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, L.; Qiu, T.; Tan, H.; Wang, Y.; Liu, W.; Li, Y. Efficient Conversion of Biomass to Formic Acid Coupled with Low Energy Consumption Hydrogen Production from Water Electrolysis. Angew. Chem. 2023, 135, e202305843. [Google Scholar] [CrossRef]





| Materials | Ultimate Analysis (daf Basis) | ||||
|---|---|---|---|---|---|
| Carbon (wt.%) | Hydrogen (wt.%) | Oxygen (wt.%) | Nitrogen (wt.%) | Ref. | |
| Glucose | 40.01 | 6.71 | 53.28 | - | |
| Starch | 44.26 | 6.82 | 48.93 | - | [50] |
| Lignin | 64.50 | 5.80 | 27.70 | 0.20 | |
| Cellulose | 44.02 | 6.40 | 49.52 | 0.03 | |
| Biomass Components | Applied Voltage (V) | Electrolysis Operating Temperature | Current Density (mA cm−2) |
|---|---|---|---|
| Glucose | 1.2 | Ambient (19 °C) | 12.97 |
| Starch | 12.48 | ||
| Lignin | 11.44 | ||
| Cellulose | 7.37 |
| Biomass Components | Applied Voltage (V) | Electrolysis Operating Temperature (°C) | Current Density (mA cm−2) |
|---|---|---|---|
| Glucose | 1.2 | 30 | 14.93 |
| 50 | 15.71 | ||
| 80 | 15.78 | ||
| Starch | 1.2 | 30 | 12.77 |
| 50 | 13.09 | ||
| 80 | 14.36 | ||
| Lignin | 1.2 | 30 | 12.48 |
| 50 | 12.84 | ||
| 80 | 13.82 | ||
| Cellulose | 1.2 | 30 | 10.15 |
| 50 | 10.28 | ||
| 80 | 12.78 |
| Feedstocks | Electrolysis Operating Temperature (°C) | H2 Yield (mL) | H2 Yield (mL/gram of Biomass) * | Ratio of Power to H2 Yield | |
|---|---|---|---|---|---|
| kWh | kWh/kg | ||||
| Glucose | Ambient (19 °C) | 12.1 | 12.1 | 3.1128 × 10−5 | 30.99 |
| 30 | 11.8 | 11.8 | 3.5832 × 10−5 | 37.96 | |
| 50 | 11.2 | 11.2 | 3.7704 × 10−5 | 44.89 | |
| 80 | 10 | 10 | 3.7872 × 10−5 | 54.89 | |
| Starch | Ambient (19 °C) | 11.4 | 11.4 | 2.9952 × 10−5 | 31.66 |
| 30 | 10.2 | 10.2 | 3.0648 × 10−5 | 37.56 | |
| 50 | 9.3 | 9.3 | 3.1416 × 10−5 | 45.04 | |
| 80 | 7.6 | 7.6 | 3.4464 × 10−5 | 65.72 | |
| Lignin | Ambient (19 °C) | 9.7 | 9.7 | 2.7456 × 10−5 | 31.70 |
| 30 | 9.2 | 9.2 | 2.9952 × 10−5 | 40.70 | |
| 50 | 8.6 | 8.6 | 3.0816 × 10−5 | 47.78 | |
| 80 | 6 | 6 | 3.3168 × 10−5 | 80.12 | |
| Cellulose | Ambient (19 °C) | 6.3 | 6.3 | 1.7688 × 10−5 | 33.83 |
| 30 | 5.9 | 5.9 | 2.436 × 10−5 | 51.61 | |
| 50 | 3.9 | 3.9 | 2.4672 × 10−5 | 84.35 | |
| 80 | 2.3 | 2.3 | 3.0672 × 10−5 | 151.54 | |
| Electrolyser | Feedstock | Catalyst | Current Density (mAcm−2) | Voltage (V) | Temp (°C) | H2 Yield (mL/h) | Ref. |
|---|---|---|---|---|---|---|---|
| H-type PEMEC | Corn straw | Poly-oxometalate (PMo12) | 20 | 0–1.20 | 80 | 7.86 | [17] |
| H-type PEMEC | Corn-stalk | FeCl3 | 10 | 0–1.20 | 80 | 4.10 | [44] |
| H-type PEMEC | Lignin | Poly-oxometalate (PMo12) | 20 | 0–1.20 | 80 | 7.92 | [51] |
| H-type PEMEC | Glucose, Starch, lignin and Cellulose | FeCl3 | 12.97, 11.4, 9.7, 6.30 | 0–1.20 | Ambient (19 °C) | 6.05, 5.7, 4.85, 3.15 | This work |
| Biomass Fuel | Pretreatment | CO2 Generation (mL) | Carbon Utilised (wt.%) | Initial Weight of Biomass Solution (g) | Final Weight of Biomass Solution (g) | |
|---|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | |||||
| Glucose | 100 | 6 | 51 | 13 | 57.55 | 57.45 |
| Starch | 36 | 8.2 | 57.51 | 57.44 | ||
| Lignin | 34 | 5.3 | 57.56 | 57.50 | ||
| Cellulose | 13.5 | 3.3 | 57.49 | 57.46 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umer, M.; Brandoni, C.; Jaffar, M.; Hewitt, N.J.; Dunlop, P.; Zhang, K.; Huang, Y. An Experimental Investigation of Hydrogen Production through Biomass Electrolysis. Processes 2024, 12, 112. https://doi.org/10.3390/pr12010112
Umer M, Brandoni C, Jaffar M, Hewitt NJ, Dunlop P, Zhang K, Huang Y. An Experimental Investigation of Hydrogen Production through Biomass Electrolysis. Processes. 2024; 12(1):112. https://doi.org/10.3390/pr12010112
Chicago/Turabian StyleUmer, Muhammad, Caterina Brandoni, Mohammad Jaffar, Neil J. Hewitt, Patrick Dunlop, Kai Zhang, and Ye Huang. 2024. "An Experimental Investigation of Hydrogen Production through Biomass Electrolysis" Processes 12, no. 1: 112. https://doi.org/10.3390/pr12010112
APA StyleUmer, M., Brandoni, C., Jaffar, M., Hewitt, N. J., Dunlop, P., Zhang, K., & Huang, Y. (2024). An Experimental Investigation of Hydrogen Production through Biomass Electrolysis. Processes, 12(1), 112. https://doi.org/10.3390/pr12010112