Functional Improvement of NiOx/CeO2 Model Catalyst Active in Dry Methane Reforming via Optimization of Nickel Content
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structural Characterization
3.2. Morphology
3.3. Surface Characterization
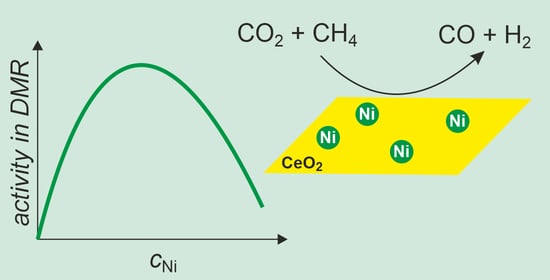
3.4. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, C. Global Challenges and Strategies for Control, Conversion and Utilization of CO2 for Sustainable Development Involving Energy, Catalysis, Adsorption and Chemical Processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Usman, M.; Wan Daud, W.M.A.; Abbas, H.F. Dry Reforming of Methane: Influence of Process Parameters—A Review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Chen, M.; Liang, D.; Yang, Z.; Cheng, W.; Tang, Z.; Wang, J.; Zhang, H. Recent Advances during CH4 Dry Reforming for Syngas Production: A Mini Review. Int. J. Hydrogen Energy 2021, 46, 5852–5874. [Google Scholar] [CrossRef]
- Fisher, F.; Tropsch, H. Conversion of Methane into Hydrogen and Carbon Monoxide. Brennst.-Chem. 1928, 9, 39–46. [Google Scholar]
- Zhang, G.; Liu, J.; Xu, Y.; Sun, Y. A Review of CH4–CO2 Reforming to Synthesis Gas over Ni-Based Catalysts in Recent Years (2010–2017). Int. J. Hydrogen Energy 2018, 43, 15030–15054. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An Overview on Dry Reforming of Methane: Strategies to Reduce Carbonaceous Deactivation of Catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Lang, J. Experimentelle Beiträge zur Kenntnis der Vorgänge bei der Wasser- und Heizgasbereitung. Z. Phys. Chem. 2017, 2U, 161–183. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst Design for Dry Reforming of Methane: Analysis Review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Shahul Hamid, M.Y. A Review on Catalyst Development for Dry Reforming of Methane to Syngas: Recent Advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Guan, Y.; Song, G.; Li, C.; Lim, K.H.; Wang, B.; Xia, L.; Song, H.; Liu, Y.; Wu, C.; Kawi, S. Ni-Based Core-Shell Structured Catalysts for Efficient Conversion of CH4 to H2: A Review. Carbon Capture Sci. Technol. 2024, 11, 100200. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, H.; Zhang, M.; Liang, C.; Duan, L. Recent Advances in Promoting Dry Reforming of Methane Using Nickel-Based Catalysts. Catal. Sci. Technol. 2024, 14, 1712–1729. [Google Scholar] [CrossRef]
- Xu, Z.; Park, E.D. Recent Advances in Coke Management for Dry Reforming of Methane over Ni-Based Catalysts. Catalysts 2024, 14, 176. [Google Scholar] [CrossRef]
- Liu, S.; Dun, C.; Jiang, Q.; Xuan, Z.; Yang, F.; Guo, J.; Urban, J.J.; Swihart, M.T. Challenging Thermodynamics: Combining Immiscible Elements in a Single-Phase Nano-Ceramic. Nat. Commun. 2024, 15, 1167. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, J.; Kim, Y.J.; Youn, J.R.; Kim, D.H.; Shapiro, D.; Guo, J.; Lee, K. Surface Control of Ni-Al2O3 Dry Reforming of Methane Catalyst by Composition Segregation. J. CO2 Util. 2024, 81, 102721. [Google Scholar] [CrossRef]
- Bai, X.; Yao, X.; Cheng, Q.; Mohamed, H.O.; Telalovic, S.; Melinte, G.A.; Emwas, A.H.; Gascon, J.; Castaño, P. Engineering the TiOx Overlayer on Ni Catalyst to Balance Conversion and Stability for Methane Dry-CO2 Reforming. ACS Sustain. Chem. Eng. 2024, 12, 633–644. [Google Scholar] [CrossRef]
- Kaviani, M.; Rezaei, M.; Alavi, S.M.; Akbari, E. Biogas Dry Reforming over Nickel-Silica Sandwiched Core–Shell Catalysts with Various Shell Thicknesses. Fuel 2024, 355, 129533. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Patel, N.; Srivastava, V.K.; Osman, A.I.; Rooney, D.W.; Fakeeha, A.H.; Abasaeed, A.E.; Alotibi, M.F.; Kumar, R. Iron-Promoted Zirconia-Alumina Supported Ni Catalyst for Highly Efficient and Cost-Effective Hydrogen Production via Dry Reforming of Methane. J. Environ. Sci. 2025, 148, 274–282. [Google Scholar] [CrossRef]
- Elnour, A.Y.; Fakeeha, A.H.; Ibrahim, A.A.; Osman, A.I.; Abasaeed, A.E.; Adil, S.F.; Kumar, R.; Al-Fatesh, A.S. Enhancing Catalytic Performance, Coke Resistance, and Stability with Strontium-Promoted Ni/WO3-ZrO2 Catalysts for Methane Dry Reforming. Res. Chem. Intermed. 2024, 50, 1211–1230. [Google Scholar] [CrossRef]
- Abahussain, A.A.M.; Al-Fatesh, A.S.; Singh, S.K.; Almutairi, G.; Fakeeha, A.H.; Ibrahim, A.A.; Abasaeed, A.E.; Frusteri, L.; Labhasetwar, N.K. Cs Promoted Ni/ZrO2-Al2O3 Catalysts for Dry Reforming of Methane: Promotional Effects of Cs for Enhanced Catalytic Activity and Stability. Arab. J. Chem. 2024, 17, 105564. [Google Scholar] [CrossRef]
- Horváth, A.; Németh, M.; Beck, A.; Sáfrán, G.; La Parola, V.; Liotta, L.F.; Žerjav, G.; Roškarič, M.; Pintar, A. Longevity Increase of an Impregnated Ni/CeO2-Al2O3 Dry Reforming Catalyst by Indium. Appl. Catal. A Gen. 2024, 669, 1167. [Google Scholar] [CrossRef]
- Liang, D.; Wang, Y.; Xu, W.; Chen, M.; Wang, M.; Li, C.; Wang, J.; Yang, Z.; Yuan, L. Ni-Pt-Co Clay Catalysts for Dry Reforming of Methane: Influence of Trimetallic Interfaces and Ce Addition. Mol. Catal. 2024, 552, 113658. [Google Scholar] [CrossRef]
- Khatun, R.; Pal, R.S.; Shoeb, M.A.; Khurana, D.; Singhl, S.; Siddiqui, N.; Poddar, M.K.; Khan, T.S.; Bal, R. Generation of Active Oxygen Species by CO2 Dissociation over Defect-Rich Ni-Pt/CeO2 Catalyst for Boosting Methane Activation in Low-Temperature Dry Reforming: Experimental and Theoretical Study. Appl. Catal. B Environ. 2024, 340, 123243. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Bagabas, A.A.; Lanre, M.S.; Osman, A.I.; Kasim, S.O.; Ibrahim, A.A.; Arasheed, R.; Alkhalifa, A.; Elnour, A.Y.; Abasaeed, A.E.; et al. Catalytic Performance of Metal Oxides Promoted Nickel Catalysts Supported on Mesoporous γ-Alumina in Dry Reforming of Methane. Processes 2020, 8, 522. [Google Scholar] [CrossRef]
- Shang, Z.; Li, S.; Li, L.; Liu, G.; Liang, X. Highly Active and Stable Alumina Supported Nickel Nanoparticle Catalysts for Dry Reforming of Methane. Appl. Catal. B Environ. J. 2017, 201, 302–309. [Google Scholar] [CrossRef]
- Bacariza, C.; Karam, L.; El Hassan, N.; Lopes, J.M.; Henriques, C. Carbon Dioxide Reforming of Methane over Nickel-Supported Zeolites: A Screening Study. Processes 2022, 10, 1331. [Google Scholar] [CrossRef]
- Moradi, G.; Khezeli, F.; Hemmati, H. Syngas Production with Dry Reforming of Methane over Ni/ZSM-5 Catalysts. J. Nat. Gas Sci. Eng. 2016, 33, 657–665. [Google Scholar] [CrossRef]
- Muñoz, H.J.; Korili, S.A.; Gil, A. Facile Synthesis of an Ni/LaAlO3—Perovskite via an MOF Gel Precursor for the Dry Reforming of Methane. Catal. Today 2024, 429, 114487. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Xiao, Y.S.; Liu, Z.W. NiO-MgO Prepared by the Complex-Decomposition Method as a Catalyst for Carbon Dioxide Reforming of Methane. Processes 2023, 11, 596. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Wongsakulphasatch, S.; Vo, D.V.N. Understanding the Role of Surface Basic Sites of Catalysts in CO2 Activation in Dry Reforming of Methane: A Short Review. Catal. Sci. Technol. 2020, 10, 35–45. [Google Scholar] [CrossRef]
- Adamski, A.; Legutko, P.; Dziadek, K.; Michalik, M.; Sadykov, V.A. Dry Reforming of Methane on Supported Catalysts Based on Nickel as an Example of Technology Limiting Emission Level of Greenhouse Gases. Przem. Chem. 2019, 98, 1158–1161. [Google Scholar] [CrossRef]
- Adamski, A.; Legutko, P.; Dziadek, K.; Parkhomenko, K.; Aymonier, C.; Sadykov, V.A.; Roger, A.-C. Role of CeO2-ZrO2 Support for Structural, Textural and Functional Properties of Ni-Based Catalysts Active in Dry Reforming of Methane. E3S Web Conf. 2019, 108, 02018. [Google Scholar] [CrossRef]
- Auxéméry, A.; Frias, B.B.; Smal, E.; Dziadek, K.; Philippot, G.; Legutko, P.; Simonov, M.; Thomas, S.; Adamski, A.; Sadykov, V.; et al. Continuous Supercritical Solvothermal Preparation of Nanostructured Ceria-Zirconia as Supports for Dry Methane Reforming Catalysts. J. Supercrit. Fluids 2020, 162, 104855. [Google Scholar] [CrossRef]
- Tu, P.H.; Le, D.N.; Dao, T.D.; Tran, Q.T.; Doan, T.C.D.; Shiratori, Y.; Dang, C.M. Paper-Structured Catalyst Containing CeO2–Ni Flowers for Dry Reforming of Methane. Int. J. Hydrogen Energy 2020, 45, 18363–18375. [Google Scholar] [CrossRef]
- Pizzolitto, C.; Pupulin, E.; Menegazzo, F.; Ghedini, E.; Di Michele, A.; Mattarelli, M.; Cruciani, G.; Signoretto, M. Nickel Based Catalysts for Methane Dry Reforming: Effect of Supports on Catalytic Activity and Stability. Int. J. Hydrogen Energy 2019, 44, 28065–28076. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Influence of Ce/Zr Molar Ratio on Catalytic Performance of Hydrotalcite-Derived Catalysts at Low Temperature CO2 Methane Reforming. Int. J. Hydrogen Energy 2017, 42, 23556–23567. [Google Scholar] [CrossRef]
- Liu, H.; Da Costa, P.; Hadj Taief, H.B.; Benzina, M.; Gálvez, M.E. Ceria and Zirconia Modified Natural Clay Based Nickel Catalysts for Dry Reforming of Methane. Int. J. Hydrogen Energy 2017, 42, 23508–23516. [Google Scholar] [CrossRef]
- Lin, K.-H.; Chang, H.-F.; Chang, A.C.-C. Biogas Reforming for Hydrogen Production over Mesoporous Ni2xCe1−xO2 Catalysts. Int. J. Hydrogen Energy 2012, 37, 15696–15703. [Google Scholar] [CrossRef]
- Lanre, M.S.; Al-Fatesh, A.S.; Fakeeha, A.H.; Kasim, S.O.; Ibrahim, A.A.; Al-Awadi, A.S.; Al-Zahrani, A.A.; Abasaeed, A.E. Catalytic Performance of Lanthanum Promoted Ni/ZrO2 for Carbon Dioxide Reforming of Methane. Processes 2020, 8, 1502. [Google Scholar] [CrossRef]
- Osojnik Črnivec, I.G.; Djinović, P.; Erjavec, B.; Pintar, A. Effect of Synthesis Parameters on Morphology and Activity of Bimetallic Catalysts in CO2-CH4 Reforming. Chem. Eng. J. 2012, 207–208, 299–307. [Google Scholar] [CrossRef]
- Aw, M.S.; Osojnik Črnivec, I.G.; Pintar, A. Toward Enhanced Conversion of Model Biogas Mixtures: Parametric Tuning and Mechanistic Study for Ceria-Zirconia Supported Nickel-Cobalt Catalyst. Catal. Sci. Technol. 2014, 4, 1340–1349. [Google Scholar] [CrossRef]
- Newnham, J.; Mantri, K.; Amin, M.H.; Tardio, J.; Bhargava, S.K. Highly Stable and Active Ni-Mesoporous Alumina Catalysts for Dry Reforming of Methane. Int. J. Hydrogen Energy 2012, 37, 1454–1464. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A.K. Effects of Metal Content on Activity and Stability of Ni-Co Bimetallic Catalysts for CO2 Reforming of CH4. Appl. Catal. A Gen. 2008, 339, 121–129. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, K.I.; Kim, D.; Song, I.K. Effect of Promoters in the Methane Reforming with Carbon Dioxide to Synthesis Gas over Ni/HY Catalysts. J. Mol. Catal. A Chem. 2006, 246, 43–48. [Google Scholar] [CrossRef]
- Lee, J.A.; Bae, Y.; Hong, K.; Hong, J. Comparative Evaluation of Ni-Based Bimetallic Catalysts for Dry Reforming of Methane at Low Temperature: The Effect of Alloy Itself on Performance. Int. J. Energy Res. 2022, 46, 11228–11249. [Google Scholar] [CrossRef]
- Abdelsadek, Z.; Köten, H.; Gonzalez-Cortes, S.; Cherifi, O.; Halliche, D.; Masset, P.J. Lanthanum-Promoted Nickel-Based Catalysts for the Dry Reforming of Methane at Low Temperatures. JOM 2023, 75, 727–738. [Google Scholar] [CrossRef]
- Lyu, Y.; Jocz, J.; Xu, R.; Stavitski, E.; Sievers, C. Nickel Speciation and Methane Dry Reforming Performance of Ni/CexZr1- XO2 Prepared by Different Synthesis Methods. ACS Catal. 2020, 10, 11235–11252. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachrichten Von Der Ges. Der Wiss. Zu Göttingen Math. Kl. 1918, 1918, 98–100. [Google Scholar]
- André, R.S.; Zanetti, S.M.; Varela, J.A.; Longo, E. Synthesis by a Chemical Method and Characterization of CaZrO3 Powders: Potential Application as Humidity Sensors. Ceram. Int. 2014, 40, 16627–16634. [Google Scholar] [CrossRef]
- Martín-Espejo, J.L.; Merkouri, L.P.; Gándara-Loe, J.; Odriozola, J.A.; Reina, T.R.; Pastor-Pérez, L. Nickel-Based Cerium Zirconate Inorganic Complex Structures for CO2 Valorisation via Dry Reforming of Methane. J. Environ. Sci. 2024, 140, 12–23. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Kasim, S.O.; Ibrahim, A.A.; Abasaeed, A.E.; Al-Fatesh, A.S. Influence of Nature Support on Methane and CO2 Conversion in a Dry Reforming Reaction over Nickel-Supported Catalysts. Materials 2019, 12, 1777. [Google Scholar] [CrossRef]
- Mo, L.; Fei, J.; Huang, C.; Zheng, X. Reforming of Methane with Oxygen and Carbon Dioxide to Produce Syngas over a Novel Pt/CoAl2O4/Al2O3 Catalyst. J. Mol. Catal. A Chem. 2003, 193, 177–184. [Google Scholar] [CrossRef]
- Yang, M.; Shen, G.; Wang, Q.; Deng, K.; Liu, M.; Chen, Y.; Gong, Y.; Wang, Z. Roles of Oxygen Vacancies of CeO2 and Mn-Doped CeO2 with the Same Morphology in Benzene Catalytic Oxidation. Molecules 2021, 26, 6363. [Google Scholar] [CrossRef] [PubMed]
- Gómez, D.A.; Coello, J.; Maspoch, S. The Influence of Particle Size on the Intensity and Reproducibility of Raman Spectra of Compacted Samples. Vib. Spectrosc. 2019, 100, 48–56. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, T.; Chen, X.; He, Y.; Liang, X. Model Construction of Micro-Pores in Shale: A Case Study of Silurian Longmaxi Formation Shale in Dianqianbei Area, SW China. Pet. Explor. Dev. 2018, 45, 412–421. [Google Scholar] [CrossRef]
- Saw, E.T.; Oemar, U.; Ang, M.L.; Kus, H.; Kawi, S. High-Temperature Water Gas Shift Reaction on Ni–Cu/CeO2 Catalysts: Effect of Ceria Nanocrystal Size on Carboxylate Formation. Catal. Sci. Technol. 2016, 6, 5336–5349. [Google Scholar] [CrossRef]
- Natile, M.M.; Boccaletti, G.; Glisenti, A. Properties and Reactivity of Nanostructured CeO2 Powders: Comparison among Two Synthesis Procedures. Chem. Mater. 2005, 17, 6272–6286. [Google Scholar] [CrossRef]
- Chen, K.; Li, W.; Guo, G.; Zhu, C.; Wu, W.; Yuan, L. Nickel Hydroxide Nanosheets Prepared by a Direct Manual Grinding Strategy for High-Efficiency Catalytic Combustion of Methane. ACS Omega 2022, 7, 8536–8546. [Google Scholar] [CrossRef] [PubMed]
- Bensalem, A.; Muller, J.C.; Bozon-Verduraz, F. Faraday Communications. From Bulk CeO2 to Supported Cerium–Oxygen Clusters: A Diffuse Reflectance Approach. J. Chem. Soc. Faraday Trans. 1992, 88, 153–154. [Google Scholar] [CrossRef]
- Guo, M.; Lu, J.; Wu, Y.; Wang, Y.; Luo, M. UV and Visible Raman Studies of Oxygen Vacancies in Rare-Earth-Doped Ceria. Langmuir 2011, 27, 3872–3877. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 Catalysts for the Dry Reforming of Methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray Photoelectron Spectroscopic Chemical State Quantification of Mixed Nickel Metal, Oxide and Hydroxide Systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Babakouhi, R.; Alavi, S.M.; Rezaei, M.; Akbari, E.; Varbar, M. Combined CO2 Reforming and Partial Oxidation of Methane over Mesoporous Nanostructured Ni/M-Al2O3 Catalyst: Effect of Various Support Promoters and Nickel Loadings. J. CO2 Util. 2023, 70, 102427. [Google Scholar] [CrossRef]
- Dedov, A.G.; Loktev, A.S.; Arkhipova, V.A.; Bykov, M.A.; Sadovnikov, A.A.; Cherednichenko, K.A.; Shandryuk, G.A. A New Approach to the Preparation of Stable Oxide-Composite Cobalt–Samarium Catalysts for the Production of Hydrogen by Dry Reforming of Methane. Processes 2023, 11, 2296. [Google Scholar] [CrossRef]
- Shtyka, O.; Zakrzewski, M.; Ciesielski, R.; Kedziora, A.; Dubkov, S.; Ryazanov, R.; Szynkowska, M.; Maniecki, T. Efficient Removal of the Carbon Deposits Formed during the Mixed Methane Reforming over Ni/Al2O3. Korean J. Chem. Eng. 2020, 37, 209–215. [Google Scholar] [CrossRef]
- Stanley, K.; Kelly, S.; Sullivan, J.A. Effect of Ni NP Morphology on Catalyst Performance in Non-Thermal Plasma-Assisted Dry Reforming of Methane. Appl. Catal. B Environ. 2023, 328, 122533. [Google Scholar] [CrossRef]









| Sample | Ni/at. % | Ce/at. % | O/at. % |
|---|---|---|---|
| CeO2 | - | 18 | 82 |
| 0.2NiOx/CeO2 | 2.1 | 18 | 80 |
| 1NiOx/CeO2 | 3.8 | 16 | 80 |
| 3NiOx/CeO2 | 4.7 | 16 | 79 |
| 5NiOx/CeO2 | 6.1 | 15 | 79 |
| 10NiOx/CeO2 | 6.3 | 13 | 81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legutko, P.; Marzec, M.; Kozieł, M.; Sokołowski, K.; Michalik, M.; Adamski, A. Functional Improvement of NiOx/CeO2 Model Catalyst Active in Dry Methane Reforming via Optimization of Nickel Content. Processes 2024, 12, 851. https://doi.org/10.3390/pr12050851
Legutko P, Marzec M, Kozieł M, Sokołowski K, Michalik M, Adamski A. Functional Improvement of NiOx/CeO2 Model Catalyst Active in Dry Methane Reforming via Optimization of Nickel Content. Processes. 2024; 12(5):851. https://doi.org/10.3390/pr12050851
Chicago/Turabian StyleLegutko, Piotr, Mateusz Marzec, Marcin Kozieł, Krystian Sokołowski, Marek Michalik, and Andrzej Adamski. 2024. "Functional Improvement of NiOx/CeO2 Model Catalyst Active in Dry Methane Reforming via Optimization of Nickel Content" Processes 12, no. 5: 851. https://doi.org/10.3390/pr12050851
APA StyleLegutko, P., Marzec, M., Kozieł, M., Sokołowski, K., Michalik, M., & Adamski, A. (2024). Functional Improvement of NiOx/CeO2 Model Catalyst Active in Dry Methane Reforming via Optimization of Nickel Content. Processes, 12(5), 851. https://doi.org/10.3390/pr12050851