Rheology of Green Plasticizer/Poly(vinyl chloride) Blends via Time–Temperature Superposition
Abstract
:1. Introduction
2. Materials and Methods
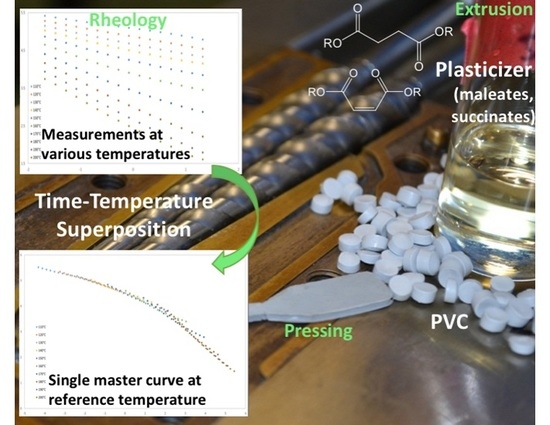
2.1. Extrusion of PVC–Plasticizer Blends
2.2. Preparation of Test Disks
2.3. Rheology
2.4. Time–Temperature Superposition
2.5. Statistics
3. Results
4. Discussion
4.1. Dynamic Rheological Response
4.2. Evaluation of Plasticizers
4.3. Succinate and Maleate Plasticizers
4.4. Dibenzoate Plasticizers
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mishra, M.; Yagci, Y. Handbook of Vinyl Polymers: Radical Polymerization, Process, and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Wilkes, C.; Summers, J.W.; Daniels, C.A. Pvc handbook; Hanser Gardner Publications: Cincinnati, OH, USA, 2005. [Google Scholar]
- Cullen, S. Global plasticizer update. In Proceedings of the SPI 23rd Annual Flexible Vinyl Products Conference, Annapolis, MD, USA, 16 July 2012; p. 4. [Google Scholar]
- Hester, R.E.; Harrison, R.M. Chemical Alternatives Assessments; Royal Society of Chemistry: London, UK, 2013. [Google Scholar]
- Kastner, J.; Cooper, D.G.; Marić, M.; Dodd, P.; Yargeau, V. Aqueous leaching of di-2-ethylhexyl phthalate and “green” plasticizers from poly (vinyl chloride). Sci. Total Environ. 2012, 432, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Wams, T. Diethylhexylphthalate as an environmental contaminant—A review. Sci. Total Environ. 1987, 66, 1–16. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Maric, M.; Nicell, J.A.; Leask, R.L.; Yargeau, V. Leaching of the plasticizer di (2-ethylhexyl) phthalate (dehp) from plastic containers and the question of human exposure. Appl. Microbiol. Biotechnol. 2014, 98, 9967–9981. [Google Scholar] [CrossRef] [PubMed]
- Feige, J.N.; Gelman, L.; Rossi, D.; Zoete, V.; Métivier, R.; Tudor, C.; Anghel, S.I.; Grosdidier, A.; Lathion, C.; Engelborghs, Y. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor γ modulator that promotes adipogenesis. J. Biol. Chem. 2007, 282, 19152–19166. [Google Scholar] [CrossRef] [PubMed]
- Latini, G.; Verrotti, A.; De Felice, C. Di-2-ethylhexyl phthalate and endocrine disruption: A review. Curr. Drug Targets Immune Endocr. Metabol. Disord. 2004, 4, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Proposal for Legislative Action on di(2-ethylhexyl) Phthalate under the Hazardous Products Act; Health Canada: Ottawa, ON, Canada, 2007.
- Consumer Product Safety Improvement Act of 2008; Public law No. 110–314 (hr4040); U.S. Congress: Washington, DC, USA, 2008.
- Commission Regulation (ec) No 552/2009 of 22 June 2009 Amending Regulation (ec) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (Reach) as Regards Annex Xvii (Text with Eea Relevance); European Union: Maastricht, The Netherlands, 2009.
- Wadey, B.L. An innovative plasticizer for sensitive applications. J. Vinyl Addit. Technol. 2003, 9, 172–176. [Google Scholar] [CrossRef]
- Lott, S. Phthalate-Free Plasticizers in Pvc; Healthy Building Network: Washington, DC, USA, 2014; pp. 1–26. [Google Scholar]
- Firlotte, N.; Cooper, D.G.; Marić, M.; Nicell, J.A. Characterization of 1,5-pentanediol dibenzoate as a potential “green” plasticizer for poly (vinyl chloride). J. Vinyl Addit. Technol. 2009, 15, 99–107. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Dodd, P.; Leask, R.L.; Maric, M.; Cooper, D.G. Designing green plasticizers: Influence of alkyl chain length on biodegradation and plasticization properties of succinate based plasticizers. Chemosphere 2013, 91, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Erythropel, H.C.; Brown, T.; Maric, M.; Nicell, J.A.; Cooper, D.G.; Leask, R.L. Designing greener plasticizers: Effects of alkyl chain length and branching on the biodegradation of maleate based plasticizers. Chemosphere 2015, 134, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Erythropel, H.C.; Shipley, S.; Börmann, A.; Nicell, J.A.; Maric, M.; Leask, R.L. Designing green plasticizers: Influence of molecule geometry and alkyl chain length on the plasticizing effectiveness of diester plasticizers in pvc blends. Polymer 2016, 89, 18–27. [Google Scholar] [CrossRef]
- Nardelli, T.C.; Erythropel, H.C.; Robaire, B. Toxicogenomic screening of replacements for di (2-ethylhexyl) phthalate (dehp) using the immortalized tm4 sertoli cell line. PLoS ONE 2015, 10, e0138421. [Google Scholar] [CrossRef] [PubMed]
- Segura, P.A.; Kaplan, P.; Erythropel, H.C.; Yargeau, V. Comparative rapid toxicity screening of commercial and potential “green” plasticizers using bioluminescent bacteria. Ind. Eng. Chem. Res. 2012, 51, 11555–11560. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Maric, M.; Cooper, D.G. Designing green plasticizers: Influence of molecular geometry on biodegradation and plasticization properties. Chemosphere 2012, 86, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Chanda, M.; Roy, S.K. Plastics Technology Handbook; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Ferry, J.D. Viscoelastic Properties of Polymers; Wiley: Hoboken, NJ, USA, 1980. [Google Scholar]
- Zou, J.; Su, L.; You, F.; Chen, G.; Guo, S. Dynamic rheological behavior and microcrystalline structure of dioctyl phthalate plasticized poly (vinyl chloride). J. Appl. Polym. Sci. 2011, 121, 1725–1733. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook: For Users of Rotational and Oscillatory Rheometers; Vincentz Network GmbH & Co KG: Hannover, Germany, 2006. [Google Scholar]
- Maiza, M.; Benaniba, M.T.; Quintard, G.; Massardier-Nageotte, V. Biobased additive plasticizing polylactic acid (pla). Polímeros 2015, 25, 581–590. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Epoxidized vegetable oils plasticized poly (lactic acid) biocomposites: Mechanical, thermal and morphology properties. Molecules 2014, 19, 16024–16038. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Hida, H.; Taniguchi, T.; Koyama, K.; Aoki, Y. Rheological properties of poly (vinyl chloride)/plasticizer systems—relation between sol–gel transition and elongational viscosity. Rheol. Acta 2007, 46, 957–964. [Google Scholar] [CrossRef]
- Van Gurp, M.; Palmen, J. Time-temperature superposition for polymeric blends. Rheol. Bull 1998, 67, 5–8. [Google Scholar]
- Dealy, J.; Plazek, D. Time-temperature superposition—a users guide. Rheol. Bull. 2009, 78, 16–31. [Google Scholar]
- Witenhafer, D. Infrared studies of crystallinity in poly (vinyl chloride). J. Macromol. Sci. Part B 1970, 4, 915–929. [Google Scholar] [CrossRef]
- Zarraga, A.; Pena, J.; Munoz, M.; Santamaria, A. Thermorheological analysis of pvc blends. J. Polym. Sci. Part B 2000, 38, 469–477. [Google Scholar] [CrossRef]
- Chazeau, L.; Paillet, M.; Cavaille, J. Plasticized pvc reinforced with cellulose whiskers. I. Linear viscoelastic behavior analyzed through the quasi-point defect theory. J. Polym. Sci. Part B 1999, 37, 2151–2164. [Google Scholar] [CrossRef]
- McKinney, P.V.; Foltz, C.R. Dta evidence for physical orientation (crystallinity) in pvc. J. Appl. Polym. Sci. 1967, 11, 1189–1197. [Google Scholar] [CrossRef]
- Hata, N.; Tobolsky, A.; Bondi, A. Effect of plasticizers on the viscoelastic properties of poly (vinyl chloride). J. Appl. Polym. Sci. 1968, 12, 2597–2613. [Google Scholar] [CrossRef]
- Leuchs, O. Zur weichmachung von polyvinylchlorid. Kunststoffe 1956, 46, 547–554. [Google Scholar]
- Boisvert, A.; Jones, S.; Issop, L.; Erythropel, H.C.; Papadopoulos, V.; Culty, M. In vitro functional screening as a means to identify new plasticizers devoid of reproductive toxicity. Environ. Res. 2016, 150, 496–512. [Google Scholar] [CrossRef] [PubMed]






| Succinates | ||||
| DES | DBS | DHS | DOS | DEHS |
| ns | ns | *** (lower) | ns | ns |
| Maleates | ||||
| DEM | DBM | DHM | DOM | DEHM |
| *** (higher) | ns | ns | ns | ns |
| Dibenzoates | ||||
| 1,3-PDDB | 1,4-BDDB | 1,5-PDDB | 1,6-HDDB | |
| *** (lower) | *** (lower) | *** (lower) | *** (lower) | |
| Succinates | ||||
| DES | DBS | DHS | DOS | DEHS |
| *** (higher) | ns | *** (lower) | ns | ns |
| Maleates | ||||
| DEM | DBM | DHM | DOM | DEHM |
| *** (higher) | ns | ns | *** (lower) | ns |
| Dibenzoates | ||||
| 1,3-PDDB | 1,4-BDDB | 1,5-PDDB | 1,6-HDDB | |
| *** (lower) | *** (lower) | *** (lower) | *** (lower) | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamarani, R.; Erythropel, H.C.; Burkat, D.; Nicell, J.A.; Leask, R.L.; Maric, M. Rheology of Green Plasticizer/Poly(vinyl chloride) Blends via Time–Temperature Superposition. Processes 2017, 5, 43. https://doi.org/10.3390/pr5030043
Jamarani R, Erythropel HC, Burkat D, Nicell JA, Leask RL, Maric M. Rheology of Green Plasticizer/Poly(vinyl chloride) Blends via Time–Temperature Superposition. Processes. 2017; 5(3):43. https://doi.org/10.3390/pr5030043
Chicago/Turabian StyleJamarani, Roya, Hanno C. Erythropel, Daniel Burkat, James A. Nicell, Richard L. Leask, and Milan Maric. 2017. "Rheology of Green Plasticizer/Poly(vinyl chloride) Blends via Time–Temperature Superposition" Processes 5, no. 3: 43. https://doi.org/10.3390/pr5030043
APA StyleJamarani, R., Erythropel, H. C., Burkat, D., Nicell, J. A., Leask, R. L., & Maric, M. (2017). Rheology of Green Plasticizer/Poly(vinyl chloride) Blends via Time–Temperature Superposition. Processes, 5(3), 43. https://doi.org/10.3390/pr5030043