Influence of Organic Ligands on the Colloidal Stability and Removal of ZnO Nanoparticles from Synthetic Waters by Coagulation
Abstract
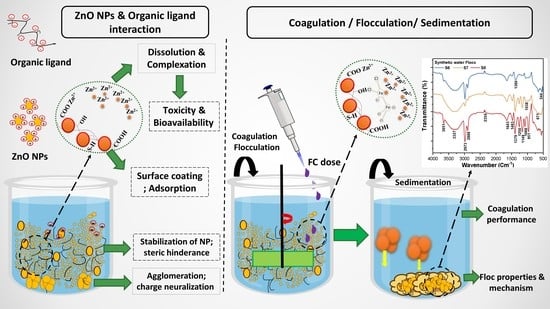
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Procedure
2.2.1. Preparation of Stock Solutions
2.2.2. Batch Sorption Experiments
2.2.3. Preparation of Synthetic Waters
2.2.4. Dissolution and Sedimentation Measurements
2.2.5. Laboratory Jar Test Experiments with Synthetic Waters
2.3. Other Analytical Methods
3. Results and Discussion
3.1. Characterization of the ZnO NPs in Aqueous Solution
3.2. Ligand Adsorption
3.3. Influence of Synthetic Waters on the Behavior of ZnO NPs
3.3.1. ζ-Potential and Particle Size of ZnO NPs
3.3.2. Dissolution and Aggregation of ZnO NPs
3.3.3. Removal of ZnO NPs and Zn2+
3.3.4. Removal of DOC and UV254
3.3.5. Characteristics of Flocs
3.4. Environmental Significance
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Keller, A.A.; Vosti, W.; Wang, H.; Lazareva, A. Release of engineered nanomaterials from personal care products throughout their life cycle. J. Nanopart. Res. 2014, 16. [Google Scholar] [CrossRef] [Green Version]
- Guo, K.; Han, F.X.; Kingery, W.; Sun, H.; Zhang, J. Development of novel nanomaterials for remediation of heavy metals and radionuclides in contaminated water. Nanotechnol. Environ. Eng. 2016, 1, 7. [Google Scholar] [CrossRef]
- Sun, T.Y.; Bornhöft, N.A.; Hungerbühler, K.; Nowack, B. Dynamic probabilistic modeling of environmental emissions of engineered nanomaterials. Environ. Sci. Technol. 2016, 50, 4701–4711. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16. [Google Scholar] [CrossRef]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14, 1109. [Google Scholar] [CrossRef]
- Gottschalk, F.; Nowack, B. The release of engineered nanomaterials to the environment. J. Environ. Monit. 2011, 13, 1145. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, E.A.; Keller, A.A.; Mädler, L.; Zhou, D.; Pokhrel, S.; Cherr, G.N. Metal oxide nanomaterials in seawater: Linking physicochemical characteristics with biological response in sea urchin development. J. Hazard. Mater. 2011, 192, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Cupi, D.; Hartmann, N.B.; Baun, A. Influence of pH and media composition on suspension stability of silver, zinc oxide, and titanium dioxide nanoparticles and immobilization of Daphnia magna under guideline testing conditions. Ecotoxicol. Environ. Saf. 2016, 127, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreher, K.L. Health and environmental impact of nanotechnology: Toxicological assessment of manufactured nanoparticles. Toxicol. Sci. 2004, 77, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Xu, Y.; Huang, C.C.; Ma, Y.; Shannon, K.B.; Chen, D.R.; Huang, Y.W. Toxicity of nano- and micro-sized ZnO particles in human lung epithelial cells. J. Nanopart. Res. 2009, 11, 25–39. [Google Scholar] [CrossRef]
- Zhou, D.; Keller, A.A. Role of morphology in the aggregation kinetics of ZnO nanoparticles. Water Res. 2010, 44, 2948–2956. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-H.; Tso, C.; Tsai, Y.; Zhuang, C.; Shih, Y. The effect of electrolytes on the aggregation kinetics of three different ZnO nanoparticles in water. Sci. Total Environ. 2015, 530, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Shen, C.; Zheng, S.; Yang, W.; Hu, H.; Liu, J.; Shi, J. Transformation of CuO Nanoparticles in the Aquatic Environment: Influence of pH, Electrolytes and Natural Organic Matter. Nanomaterials 2017, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Afshinnia, K.; Gibson, I.; Merrifield, R.; Baalousha, M. The concentration-dependent aggregation of Ag NPs induced by cystine. Sci. Total Environ. 2016, 557–558, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Aiken, G.R.; Hsu-Kim, H. Effects of natural organic matter properties on the dissolution kinetics of zinc oxide nanoparticles. Environ. Sci. Technol. 2015, 49, 11476–11484. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, R.A. Dissolution and Aggregation of Zinc Oxide Nanoparticles at Circumneutral pH; A Study of Size Effects in the Presence and Absence of Citric Acid. Master’s Thesis, University of Iowa, Iowa City, IW, USA, 2011. [Google Scholar]
- Khan, R.; Inam, M.; Zam, S.; Park, D.; Yeom, I. Assessment of Key Environmental Factors Influencing the Sedimentation and Aggregation Behavior of Zinc Oxide Nanoparticles in Aquatic Environment. Water 2018, 10, 660. [Google Scholar] [CrossRef]
- Gondikas, A.P.; Morris, A.; Reinsch, B.C.; Marinakos, S.M.; Lowry, G.V.; Hsu-Kim, H. Cysteine-induced modifications of zero-valent silver nanomaterials: Implications for particle surface chemistry, aggregation, dissolution, and silver speciation. Environ. Sci. Technol. 2012, 46, 7037–7045. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, Y.; Tang, T.; Yuan, Z.; Yu, C.-P. Removal of silver nanoparticles by coagulation processes. J. Hazard. Mater. 2013, 261, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Ye, Y.Y.; Qi, J.; Li, F.T.; Tang, Y.L. Removal of titanium dioxide nanoparticles by coagulation: Effects of coagulants, typical ions, alkalinity and natural organic matters. Water Sci. Technol. 2013, 68, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Hyung, H.; Kim, J.H. Dispersion of C60in natural water and removal by conventional drinking water treatment processes. Water Res. 2009, 43, 2463–2470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Crittenden, J.C. Stability and removal of water soluble CdTe quantum dots in water. Environ. Sci. Technol. 2007, 42, 321–325. [Google Scholar] [CrossRef]
- Abbott Chalew, T.E.; Ajmani, G.S.; Huang, H.; Schwab, K.J. Evaluating nanoparticle breakthrough during drinking water treatment. Environ. Health Perspect. 2013, 121, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, N.; Chu, Y.; Sun, Y.; Yan, H.; Han, Q. CuO nanoparticle–humic acid (CuONP–HA) composite contaminant removal by coagulation/ultrafiltration process: The application of sodium alginate as coagulant aid. Desalination 2015, 367, 265–271. [Google Scholar] [CrossRef]
- Sun, J.; Gao, B.; Zhao, S.; Li, R.; Yue, Q.; Wang, Y.; Liu, S. Simultaneous removal of nano-ZnO and Zn2+ based on transportation character of nano-ZnO by coagulation: Enteromorpha polysaccharide compound polyaluminum chloride. Environ. Sci. Pollut. Res. 2017, 24, 5179–5188. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Fisher-Power, L.M.; Shi, Z.; Cheng, T. Cu and Zn adsorption to a terrestrial sediment: Influence of solid-to-solution ratio. Chemosphere 2017, 175, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Erto, A.; Di Natale, F.; Musmarra, D.; Lancia, A. Modeling of single and competitive adsorption of cadmium and zinc onto activated carbon. Adsorption 2015, 21, 611–621. [Google Scholar] [CrossRef]
- Cheng, T.; Barnett, M.O.; Roden, E.E.; Zhuang, J. Effects of solid-to-solution ratio on uranium(VI) adsorption and its implications. Environ. Sci. Technol. 2006, 40, 3243–3247. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.S.; Corniciuc, C.; Teixeira, M.R. The effect of TiO2 nanoparticles removal on drinking water quality produced by conventional treatment C/F/S. Water Res. 2017, 109, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Elimelech, M. Chemical and physical aspects of natural organic matter (NOM) fouling of nanofiltration membranes. J. Membr. Sci. 1997, 132, 159–181. [Google Scholar] [CrossRef]
- EPA, United States Environmental Protection Agency. Enhanced Coagulation and Enhanced Precipitative Softening Guidance Manual; Disinfection Byproducts Byproducts Rule; EPA: Washington, DC, USA, 1999.
- Edzwald, J.K.; Tobiason, J.E. Enhanced coagulation: US requirements and a broader view. Water Sci. Technol. 1999, 40, 63–70. [Google Scholar] [CrossRef]
- Kato, H.; Fujita, K.; Horie, M.; Suzuki, M.; Nakamura, A.; Endoh, S.; Yoshida, Y.; Iwahashi, H.; Takahashi, K.; Kinugasa, S. Dispersion characteristics of various metal oxide secondary nanoparticles in culture medium for in vitro toxicology assessment. Toxicol. In Vitro 2010, 24, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Zhaoxia, J.I. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-F.; Zhang, J.-Y.; Shen, Z.-G.; Zhong, J.; Yun, J. Preparation and characterization of amorphous cefuroxime axetil drug nanoparticles with novel technology: High-gravity antisolvent precipitation. Ind. Eng. Chem. Res. 2006, 45, 8723–8727. [Google Scholar] [CrossRef]
- Erhayem, M.; Sohn, M. Stability studies for titanium dioxide nanoparticles upon adsorption of Suwannee River humic and fulvic acids and natural organic matter. Sci. Total Environ. 2014, 468–469, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, X.; Han, X.; Tang, Z.; Song, F.; Zhang, S.; Zhu, Y.; Guo, W.; He, Z.; Guo, Q. Colloidal stability of Fe3O4 magnetic nanoparticles differentially impacted by dissolved organic matter and cations in synthetic and naturally-occurred environmental waters. Environ. Pollut. 2018, 241, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Koneswaran, M.; Narayanaswamy, R. L-Cysteine-capped ZnS quantum dots based fluorescence sensor for Cu2+ ion. Sens. Actuators B Chem. 2009, 139, 104–109. [Google Scholar] [CrossRef]
- Tombácz, E.; Filipcsei, G.; Szekeres, M.; Gingl, Z. Particle aggregation in complex aquatic systems. Colloids Surf. A Physicochem. Eng. Asp. 1999, 151, 233–244. [Google Scholar] [CrossRef]
- Kinsinger, N.; Honda, R.; Keene, V.; Walker, S.L. Titanium Dioxide Nanoparticle Removal in Primary Prefiltration Stages of Water Treatment: Role of Coating, Natural Organic Matter, Source Water, and Solution Chemistry. Environ. Eng. Sci. 2015, 32, 292–300. [Google Scholar] [CrossRef]
- Ravindran, A.; Dhas, S.P.; Chandrasekaran, N.; Mukherjee, A. Differential interaction of silver nanoparticles with cysteine. J. Exp. Nanosci. 2013, 8, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Baalousha, M. Aggregation and disaggregation of iron oxide nanoparticles: Influence of particle concentration, pH and natural organic matter. Sci. Total Environ. 2009, 407, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Chibowski, E.; Holysz, L.; Terpilowski, K.; Wiacek, A.E. Influence of ionic surfactants and lecithin on stability of titanium dioxide in aqueous electrolyte solution. Croat. Chem. Acta 2007, 80, 395–403. [Google Scholar]
- Maximova, N.; Dahl, O. Environmental implications of aggregation phenomena: Current understanding. Curr. Opin. Colloid Interface Sci. 2006, 11, 246–266. [Google Scholar] [CrossRef]
- Taboada-Serrano, P.; Chin, C.-J.; Yiacoumi, S.; Tsouris, C. Modeling aggregation of colloidal particles. Curr. Opin. Colloid Interface Sci. 2005, 10, 123–132. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, J.; Zhao, Q.; He, S.; Ma, J. Effect of AlCl3 concentration on nanoparticle removal by coagulation. J. Environ. Sci. 2015, 38, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Liu, C.; Zhang, J.; Lin, D. Removal of dispersant-stabilized carbon nanotubes by regular coagulants. J. Environ. Sci. 2012, 24, 1364–1370. [Google Scholar] [CrossRef]
- Duan, J.; Gregory, J. Coagulation by hydrolysing metal salts. Adv. Colloid Interface Sci. 2003, 100, 475–502. [Google Scholar] [CrossRef]
- Sharp, E.L.; Jarvis, P.; Parsons, S.A.; Jefferson, B. Impact of fractional character on the coagulation of NOM. Colloids Surf. Physicochem. Eng. Asp. 2006, 286, 104–111. [Google Scholar] [CrossRef]
- Sharp, E.L.; Parson, S.A.; Jefferson, B. Coagulation of NOM: Linking character to treatment. Water Sci. Technol. 2006, 53, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Matilainen, A.; Vepsäläinen, M.; Sillanpää, M. Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interface Sci. 2010, 159, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.; Flora, J.R. V.; Park, Y.-G.; Badawy, M.; Saleh, H.; Yoon, Y. Removal of natural organic matter from potential drinking water sources by combined coagulation and adsorption using carbon nanomaterials. Sep. Purif. Technol. 2012, 95, 64–72. [Google Scholar] [CrossRef]
- Jiang, J.-Q. The role of coagulation in water treatment. Curr. Opin. Chem. Eng. 2015, 8, 36–44. [Google Scholar] [CrossRef]
- Wang, L.; Han, C.; Nadagouda, M.N.; Dionysiou, D.D. An innovative zinc oxide-coated zeolite adsorbent for removal of humic acid. J. Hazard. Mater. 2016, 313, 283–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matrajt, G.; Borg, J.; Raynal, P.I.; Djouadi, Z.; D’Hendecourt, L.; Flynn, G.J.; Deboffle, D. FTIR and Raman analyses of the Tagish Lake meteorite: Relationship with the aliphatic hydrocarbons observed in the Diffuse Interstellar Medium. Astron. Astrophys. 2004, 416, 983–990. [Google Scholar] [CrossRef]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta 2006, 573–574, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-L.; Xia, J.; Li, S.; Sun, F. Effect of natural organic matter (NOM) on Cu (II) adsorption by multi-walled carbon nanotubes: Relationship with NOM properties. Chem. Eng. J. 2012, 200, 627–636. [Google Scholar] [CrossRef]
- Inam, M.A.; Khan, R.; Park, D.R.; Lee, Y.-W.; Yeom, I.T. Removal of Sb (III) and Sb (V) by Ferric Chloride Coagulation: Implications of Fe Solubility. Water 2018, 10, 418. [Google Scholar] [CrossRef]
- Liao, Y.; Tang, X.; Yang, Q.; Chen, W.; Liu, B.; Zhao, C.; Zhai, J.; Zheng, H. Characterization of an inorganic polymer coagulant and coagulation behavior for humic acid/algae-polluted water treatment: Polymeric zinc–ferric–silicate–sulfate coagulant. RSC Adv. 2017, 7, 19856–19862. [Google Scholar] [CrossRef]
- Sandmann, A.; Kompch, A.; Mackert, V.; Liebscher, C.H.; Winterer, M. Interaction of l-cysteine with ZnO: Structure, surface chemistry, and optical properties. Langmuir 2015, 31, 5701–5711. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; He, C.; He, Y. Preparation and characterization of poly-silicic-cation coagulants by synchronous-polymerization and co-polymerization. Chem. Eng. J. 2013, 223, 869–874. [Google Scholar] [CrossRef]
- Moussas, P.A.; Zouboulis, A.I. A study on the properties and coagulation behaviour of modified inorganic polymeric coagulant—Polyferric silicate sulphate (PFSiS). Sep. Purif. Technol. 2008, 63, 475–483. [Google Scholar] [CrossRef]
- Tsyganenko, A.A.; Lamotte, J.; Saussey, J.; Lavalley, J.C. Bending vibrations of OH groups resulting from H2 dissociation on ZnO. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1989, 85, 2397–2403. [Google Scholar] [CrossRef]
- Pinotti, A.; Zaritzky, N. Effect of aluminum sulfate and cationic polyelectrolytes on the destabilization of emulsified wastes. Waste Manag. 2001, 21, 535–542. [Google Scholar] [CrossRef]










| DOC | Various Parameters of Synthetic Waters | |||||||
|---|---|---|---|---|---|---|---|---|
| Type | Concentration | Ligand Type & Water Code | DOC (mg C/L) | UV254nm (1/cm) | SUVA (L/(mmg)) | Turbidity (NTU) | EC (µS/cm) | |
| Hydrophilic SUVA < 3 | Low-Moderate (ca. < 2 mg C/L) | SA | S1 | 1.62 | 0.017 | 1.04 | 1.05 | 158 |
| L-cys | S2 | 1.60 | 0.015 | 0.93 | 1.12 | 161 | ||
| Hydrophilic SUVA < 3 | Moderate (ca. 2–3 mg C/L) | SA | S3 | 2.69 | 0.035 | 1.30 | 1.21 | 375 |
| L-cys | S4 | 2.65 | 0.031 | 1.17 | 1.17 | 371 | ||
| Hydrophobic SUVA > 4 | HA | S5 | 2.58 | 0.195 | 7.55 | 5.06 | 378 | |
| Hydrophilic SUVA < 3 | Moderate-high (ca. 6 mg C/L) | SA | S6 | 6.31 | 0.045 | 0.71 | 1.16 | 383 |
| L-cys | S7 | 6.18 | 0.037 | 0.60 | 1.20 | 382 | ||
| Hydrophobic SUVA > 4 | HA | S8 | 5.92 | 0.510 | 8.61 | 9.80 | 388 | |
| Adsorbate | Pseudo-First Order (PFO) | Pseudo-Second Order (PSO) | ||||
|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (1/h) | R2 | qe (mg/g) | k2 (g/mg/h) | R2 | |
| HA | 40.91 | 5.405 | 0.917 | 41.78 | 0.1385 | 0.976 |
| SA | 9.822 | 1.042 | 0.937 | 10.44 | 0.0632 | 0.972 |
| L-cys | 20.46 | 2.146 | 0.863 | 21.52 | 0.0681 | 0.986 |
| Ligand | Langmuir Fitting | Freundlich Fitting | ||||
|---|---|---|---|---|---|---|
| KL (L/mg) | qm (mg/g) | R2 | n | R2 | ||
| HA | 0.18 ± 0.01 | 143.37 ± 3.30 | 0.996 | 31.60 ± 5.51 | 2.58 ± 0.36 | 0.939 |
| SA | 0.31 ± 0.04 | 40.47 ± 1.48 | 0.988 | 12.37 ± 2.49 | 3.18 ± 0.66 | 0.877 |
| L-cys | 0.29 ± 0.02 | 66.05 ± 1.77 | 0.994 | 19.96 ± 3.48 | 3.17 ± 0.57 | 0.905 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, R.; Inam, M.A.; Park, D.R.; Zam Zam, S.; Shin, S.; Khan, S.; Akram, M.; Yeom, I.T. Influence of Organic Ligands on the Colloidal Stability and Removal of ZnO Nanoparticles from Synthetic Waters by Coagulation. Processes 2018, 6, 170. https://doi.org/10.3390/pr6090170
Khan R, Inam MA, Park DR, Zam Zam S, Shin S, Khan S, Akram M, Yeom IT. Influence of Organic Ligands on the Colloidal Stability and Removal of ZnO Nanoparticles from Synthetic Waters by Coagulation. Processes. 2018; 6(9):170. https://doi.org/10.3390/pr6090170
Chicago/Turabian StyleKhan, Rizwan, Muhammad Ali Inam, Du Ri Park, Saba Zam Zam, Sookyo Shin, Sarfaraz Khan, Muhammad Akram, and Ick Tae Yeom. 2018. "Influence of Organic Ligands on the Colloidal Stability and Removal of ZnO Nanoparticles from Synthetic Waters by Coagulation" Processes 6, no. 9: 170. https://doi.org/10.3390/pr6090170
APA StyleKhan, R., Inam, M. A., Park, D. R., Zam Zam, S., Shin, S., Khan, S., Akram, M., & Yeom, I. T. (2018). Influence of Organic Ligands on the Colloidal Stability and Removal of ZnO Nanoparticles from Synthetic Waters by Coagulation. Processes, 6(9), 170. https://doi.org/10.3390/pr6090170