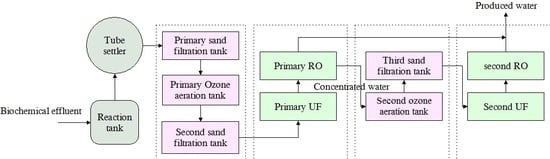
3.1. Performance of Individual Process for the Removal of Various Contaminants
Ozonation and sand filtration have been well practiced for water treatment—the former in the destruction of chemical and biological contaminants, and the latter in the removal of particulate matter [
37]. Sand filtration is a good pretreatment for UF because it removes the organic foulants from the secondary effluent and significantly increases the UF water flux [
38]. Therefore, wastewater was preprocessed by primary sand filte–ozone–secondary sand filter technique before membrane treatment. Moreover, PAC was added for the flocculation of wastewater and then precipitated before sand filtration to reduce ozone consumption. As shown in
Figure 2, the removal rate of SS was the highest (99.34 ± 0.92%), followed by color (74.01 ± 8.68%), COD
cr (39.85 ± 7.51%), NH
3-N (27.35 ± 31.78%), TP (13.25 ± 15.94%), and TN (−4.39 ± 25.02%) after treatment by the reaction, precipitation, primary sand filtration, ozonation oxidation and secondary sand filtration. SS was mainly eliminated during sand filtration. In addition, turbidity can also be eliminated during sand filtration, and the average removal rate of turbidity in the secondary sand filtration was about 37.42 ± 5.21%. The ozone dosage in the primary ozone aeration tank was approximately 90 g·m
−3 of wastewater. The average removal rates of COD
cr, color, NH
3-N, TP, and TN were 27.6 ± 7.03%, 69.4 ± 6.98%, 23.8 ± 33.33%, −18.05 ± 19.30%, and −5.63 ± 28.54%, respectively, in the primary ozone aeration tank. The color removal rate was proportional to the ozone dosage (
Figure 3). The relational expression between the ozone dosage
x and color removal rate
y was fitted with software CurveExpert 1.3 [
39] (Equation (4): correlation coefficient = 0.9957).
Figure 4 shows that the removal rates of NH
3-N, TP, and TN fluctuated violently during ozonation. The maximum removal rates of NH
3-N, TP, and TN were 87.99%, 28.65%, and 12.86%, respectively, whereas their minimum rates were −50.39%, −72.73%, and −64.40%, respectively. On the one hand, during the reaction between ozone and wastewater, either ozone oxidized some NH
3-N into NO
2-N [
40], or the supply of ozone formed bubbles that drove NH
3-N spillover in the form of NH
3. On the other hand, ozone degraded some organic nitrides in wastewater into NH
3-N through oxidation [
41]. The production of NH
3-N was accelerated when a high content of organic nitrides formed in the wastewater, thus increasing the concentration of NH
3-N in wastewater. Therefore, the removal rate of NH
3-N was negative. The removal rate of TN fluctuated for two reasons. On the one hand, some organic nitrogen particles were oxidized into inorganic nitrogen or entered into liquid phase or spillover in the form of N
2/NH
3 [
42]. On the other hand, some byproducts (e.g., NO
2) were produced during ozone preparation and were then dissolved in the wastewater [
43,
44]. Nevertheless, only a small proportion of NO
2 was produced during ozone oxidation [
43]. The removal rates of NH
3-N and TN were mainly positive and negative, respectively. This finding indicates that the organic nitrogen in wastewater slowly oxidized into N
2/NH
3. Fluctuation of TP content in wastewater could be mainly interpreted from the following two aspects. First, soluble inorganic phosphorus that mainly exist in PO
43− combined with metal ions (e.g., Ca
2+, Mg
2+, and Fe
3+) and then formed sediments. Second, parts of macromolecular organic matter in wastewater were degraded into soluble phosphorus through ozonation [
45].
Figure 5 shows that after all the treatments, the value of the wastewater attained 33–70.8 mg·L
−1 COD
cr (average of 52.02 mg·L
−1), 20–80 times color (average of 39 times), 0.15–2.02 mg·L
−1 NH
3-N (average of 0.86 mg·L
−1), 0–0.5 mg·L
−1 SS (average of 0.16 mg·L
−1), 2.73–11.9 mg·L
−1 TN (average of 6.00 mg·L
−1), and 1.47–5.68 mg·L
−1 TP (average of 3.08 mg·L
−1).
UF retains suspended particles and macromolecules. The major constituents of effluent organic matter are biopolymers, mainly soluble microbial products [
46]. Therefore, UF can eliminate COD
cr and TP to some extent but performs poorly in removing color, ammonia nitrogen, and TN [
24] (
Figure 6a,b). UF could effectively reduce turbidity but hardly influence hardness, total alkalinity, Cl
−, SO
42−, and conductivity (
Figure 6c,d). After UF treatment, the respective removal rates of COD
cr, TP, and turbidity were 17.34 ± 5.86%, 4.03 ± 3.13%, and 52.18 ± 18.07% (
Figure 7), which were lower than previously described [
24]. This finding reflected that the contents of suspended matter and macromolecular organics in wastewater might be relatively low after sand filtration + ozonation + sand filtration.
The primary RO pressure was controlled between 1.0–1.2 MPa to protect stable water yield. Pressure gradually increased upon the blockage of the RO membrane by dirt. Water yield and desalinization rate of primary RO treatment slightly changed with the increase in inlet pressure (
Table 2). The average removal rates of COD
cr, color, TP, Cl
−, SO
42−, and conductivity in the primary RO were 99.17 ± 0.88%, 99.11 ± 1.60%, 98.09 ± 1.55%, 92.02 ± 1.87%, 99.17 ± 0.81%, and 92.94 ± 2.49%, respectively. The average removal rates of total alkalinity, NH
3-N, TN, hardness, and turbidity were 87.74 ± 2.64%, 91.39 ± 2.45%, 85.07 ± 6.69%, 82.83 ± 5.19%, and 76.98 ± 5.46%, respectively (
Figure 8). The removal rate of conductivity was lower than that previously described [
47], which might have occurred because an old RO membrane was used in the primary RO system. This RO membrane had been used for 2 years. Contents of different pollutants in effluent of primary RO treatment are shown in
Table 3. COD
cr, BOD
5, SS, color, Fe
3+, and Cu
2+ were generally undetectable in RO effluent. All water quality indexes of RO effluent satisfied the reuse standards of recycled water. COD
cr, color, NH
3-N, hardness, Cl
−, SO
42−, turbidity, and contents of Fe
3+ and Cu
2+ all satisfied the standards of domestic drinking water.
To further increase the reuse rate of recycled water, we continuously purified the RO-concentrated water. Given the high contents of pollutants (COD
cr and color) in the RO-concentrated water, ozone dosage was adjusted to approximately 118 g·m
−3 of wastewater to relieve membrane blockage in the future. Contents of pollutants fluctuated slightly before and after ozone was added, indicating that ozone could hardly affect hardness, total alkalinity, Cl
−, SO
42−, and conductivity (
Figure 9). The removal rates of COD
cr and color were higher than those in the primary ozone aeration tank. The average removal rates of COD
cr and color in the secondary ozone aeration tank were 35.72 ± 6.26% and 75.36 ± 7.39%, respectively (
Figure 10). The removal rates of COD and color in the primary and secondary ozone aeration tanks were all lower than those of electrocoagulation-O
3 process, during which the ozone dose was 280 g·m
−3 and the color removal rate was close to 100% [
48].
RO concentration water was further treated by sand filtration, UF, and RO after ozonation. Given that the conductivity of RO concentrated water was very high (average of 28,236 μs·cm
−1,
Figure 10), inlet pressure of the secondary RO system was 1.5 MPa. The average removal rates of pollutants are shown in
Figure 11. The average removal rates of COD
cr, color, NH
3-N, TN, TP, hardness, total alkalinity, Cl
−, SO
42−, conductivity, and turbidity were 99.41 ± 0.63%, 99.01 ± 1.65%, 93.52 ± 2.08%, 89.11 ± 4.95%, 99.00 ± 0.79%, 98.89 ± 1.18%, 94.77 ± 2.64%, 96.34 ± 1.29%, 99.48 ± 0.41%, 97.99 ± 1.31%, and 90.17 ± 4.41%, respectively. Contents of different pollutants in effluent of the second RO treatment are listed in
Table 4. Although conductivity, total alkalinity, hardness, and contents of Cl
− and SO
42− in inflow were relatively high, the water quality indexes of the secondary RO effluent were no poorer than those of the primary RO effluent due to the use of a new RO membrane and high inlet pressure. Water quality indexes of the secondary RO effluent all satisfied the reuse standards of recycled water. Color, COD
cr, hardness, and contents of Cl
− and SO
42− all satisfied the standards of domestic drinking water.
3.2. Overall Efficiency
The combined processes showed good performance in water reuse treatment. After treatment, the overall standard rates of the COD
cr, color, NH
3-N, hardness, Cl
−, SO
42−, turbidity, Fe
3+, and Cu
2+ were −0.88, −0.98, −0.86, −0.95, −0.63, −0.94, −1 and −1, respectively, for the output water of the primary RO system and −0.85, −0.98, −0.88, −0.99, −0.57, −0.90, −0.91, −1, and −1, respectively, for the output water of the second RO system compared with the drinking water standard. The overall removal rate of COD
cr, color, NH
3-N, TN, TP, hardness, total alkalinity, Cl
−, SO
42−, conductivity, and turbidity reached 99.57%, 99.79%, 94.65%, 84.53%, 98.36%, 82.54%, 87.68%, 92.05%, 99.17%, 93.03%, and 93.18%, respectively, for the primary water reuse system and 99.62%, 99.75%, 94.75%, 88.26%, 99.12%, 98.90%, 94.83%, 96.40%, 99.48%, 97.95%, and 88.64%, respectively, for the second water reuse system (
Table 5). The RO system achieved the highest removal rate of pollutants. However, color was decreased dramatically during ozonation (92.59 and 97.27 times). The removal rate of the conductivity with new RO membranes was higher than those during UF-electrodialysis process [
22] and UF ceramic membrane [
21], and close to that during UF–diafiltration process [
19].
3.3. Energy and Chemicals Costs
Data on energy and chemicals costs for the water reuse treatment at a flow rate of 7.5 × 10
4 m
3·d
−1 (working time 24 h day
−1) are summarized in this section. Water yields in the primary RO system and secondary RO system were 50,250 and 14,850 m
3·d
−1, respectively. The reuse rate of recycled water in the whole system was 86.8%, higher than that during forward osmosis (FO)-RO system by 31.8% [
49].
For the primary ozone reaction tank, ~90 g O
3·m
−3 wastewater was consumed on average, whereas approximately 118 g O
3·m
−3 wastewater was consumed for the second ozone reaction tank. The electricity consumption of the ozone and oxygen production was 12 kWh·kg
−1 O
3 power [
3]. Thus, an operating cost of approximately 0.11 USD·m
−3 wastewater (approximately 8100 USD·d
−1 for 7.5 × 10
4 m
3·d
−1 wastewater) was calculated for a power price of 0.1 USD·kWh
−1 for the primary zone reaction tank and 0.14 USD·m
−3 wastewater (approximately 3505 USD·d
−1 for 24,750 m
3·d
−1 wastewater) for the second ozone reaction. Powers of inlet pumps in the primary UF and primary RO system were 340 kW and 2155 kW, respectively. The total power consumption of primary self-cleaning filter, sand filter, UF, and RO backwash pump was approximately 17 kW. Then, the power consumption of the pumps in the primary water reuse system was 60,288 kWh·d
−1, and the electricity cost of the pumps in the primary water reuse system was 6029 USD·d
−1. The total electricity cost in the primary water reuse system was 14,129 USD·d
−1. The power consumptions of inlet pumps in the secondary UF and secondary RO system were 110 and 770 kW, respectively. The total power consumption of secondary self-cleaning filter, sand filter, UF, and RO backwash pump was approximately 11 kW. Then, the power consumption of the pumps in the second water reuse system was 21,384 kWh·d
−1, and the electricity cost of the pumps in the second water reuse system was 2138 USD·d
−1. The total electricity cost in the second water reuse system was 5643 USD·d
−1.
The PAC dosage was approximately 80 g·m−3 of wastewater and thus the PAC cost was approximately 644 USD·d−1. The number of RO membrane and UF membrane were 5280 and 1224, respectively, and the prices of them were 500 and 2167 USD, respectively. So the costs of UF and RO membranes replacement were 53.04 × 104 and 88 × 104 USD·a−1. The number of workers in the sewage plant were 50, and the annual salary was 25,000 USD·person−1. So the employee cost was 125 × 104 USD·a−1. The Machine maintenance cost was approximately 21.91 × 104 USD·a−1. The cost of the agentia and the filter-bag were 11.42 × 104 and 3.96 × 104 USD·a−1, respectively.
The total operating cost of treating textile wastewater is displayed in
Table 6, reaching 0.44 USD·m
−3 reuse water, wherein the cost of the ozone production was 0.18 USD·m
−3 (40%), that of sand filtration, UF and RO system was 0.19 USD·m
−3 (43%), and that of machine maintenance, employee cost, agentia, and PAC was 0.08 USD·m
−3 (17%) (
Figure 12). In the filtering system, RO claimed the highest cost (0.14 USD·m
−3 reuse water), followed by UF (0.04 USD·m
−3). In RO system, the electricity cost and membrane cost were 0.11 and 0.04 USD·m
−3, respectively. The operating cost of secondary reuse system was approximately 0.086 USD·m
−3 higher than that in the primary system, which was caused by the increased ozone dosage and operating pressure of RO. Based on the above analysis, the electricity cost for ozonation and RO treatment accounted for 64.84% of the total cost. Therefore, the key to reducing the operating cost for reuse of recycled water is decreasing the electricity consumption in ozonation and RO. The operating cost of the proposed sequential system in this research was lower than that of FO-RO system in literature [
49] by 0.06 USD·m
−3 reuse water. It was also much lower than that of electrocoagulation-O
3 process, which was 5.80 USD·m
−3 treated wastewater [
48].