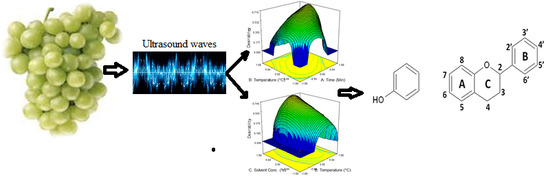
Ultrasound-Assisted Phytochemical Extraction Condition Optimization Using Response Surface Methodology from Perlette Grapes (Vitis vinifera)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extract Yield Determination
2.2. Determination of Phenolics
2.3. Determination of Flavonoids
2.4. 2,2-Diphenyl-2-picrylhydrazyl (DPPH) Assay
2.5. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Effect of Independent Variables on Extract Yield
3.2. Effect of Factors on Total Phenolic Content
3.3. Effect of Factors on Total Flavonoid Content
3.4. Effect of Factors on Antioxidant Activity
3.5. Independent Variable Optimization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rasines-Perea, Z.; Teissedre, P.-L. Grape Polyphenols’ Effects in Human Cardiovascular Diseases and Diabetes. Molecules 2017, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Duba, K.S.; Casazza, A.A.; Mohamed, H.B.; Perego, P.; Fiori, L. Extraction of polyphenols from grape skins and defatted grape seeds using subcritical water: Experiments and modeling. Food Bioprod. Process. 2015, 94, 29–38. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Ahmad, A.; Kaleem, M.; Ahmed, Z.; Shafiq, H. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections—A review. Food Res. Int. 2015, 77, 221–235. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Meneses, M.A.; Caputo, G.; Scognamiglio, M.; Reverchon, E.; Adami, R. Antioxidant phenolic compounds recovery from Mangifera indica L. by-products by supercritical antisolvent extraction. J. Food Eng. 2015, 163, 45–53. [Google Scholar] [CrossRef]
- Valdés, A.; Vidal, L.; Beltran, A.; Canals, A.; Garrigós, M.C. Microwave-assisted extraction of phenolic compounds from almond skin byproducts (Prunus amygdalus): A multivariate analysis approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef]
- Brahmi-Chendouh, N.; Piccolella, S.; Crescente, G.; Pacifico, F.; Boulekbache, L.; Hamri-Zeghichi, S.; Akkal, S.; Madani, K.; Pacifico, S. A nutraceutical extract from Inula viscosa leaves: UHPLC-HR-MS/MS based polyphenol profile, and antioxidant and cytotoxic activities. J. Food Drug Anal. 2019. [Google Scholar] [CrossRef]
- He, B.; Zhang, L.-L.; Yue, X.-Y.; Liang, J.; Jiang, J.; Gao, X.-L.; Yue, P.-X. Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, D.; Zhang, Q.-A.; Sun, D.-W. Ultrasound-assisted extraction of phenolics from wine lees: Modeling, optimization and stability of extracts during storage. Ultrason. Sonochemistry 2014, 21, 706–715. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Lester, P., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Khodaiyan, F.; Mousavi, M. Modelling and optimising of physicochemical features of walnut-oil beverage emulsions by implementation of response surface methodology: Effect of preparation conditions on emulsion stability. Food Chem. 2015, 174, 649–659. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Ghafoor, K.; Choi, Y.H.; Jeon, J.Y.; Jo, I.H. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef]
- Lapornik, B.; Prošek, M.; Wondra, A.G. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Corrales, M.; García, A.F.; Butz, P.; Tauscher, B. Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J. Food Eng. 2009, 90, 415–421. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Vieira, G.S.; Cavalcanti, R.N.; Meireles, M.A.A.; Hubinger, M.D. Chemical and economic evaluation of natural antioxidant extracts obtained by ultrasound-assisted and agitated bed extraction from jussara pulp (Euterpe edulis). J. Food Eng. 2013, 119, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Ghafoor, K.; Hui, T.; Choi, Y.H. Optimization of Ultrasonic-Assisted Extraction of Total Anthocyanins from Grape Peel Using Response Surface Methodology. J. Food Biochem. 2011, 35, 735–746. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Thermal stability of anthocyanins and colourless phenolics in pomegranate (Punica granatum L.) juices and model solutions. Food Chem. 2013, 138, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—A response surface approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar]
- Rajha, H.N.; El Darra, N.; Hobaika, Z.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Extraction of total phenolic compounds, flavonoids, anthocyanins and tannins from grape byproducts by response surface methodology. Influence of solid-liquid ratio, particle size, time, temperature and solvent mixtures on the optimization process. Food Nutr. Sci. 2014, 5, 397. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimisation of microwave-assisted extraction from Phyllanthus amarus for phenolic compounds-enriched extracts and antioxidant capacity. Chem. Pap. 2016, 70, 713–725. [Google Scholar] [CrossRef]

| - Variables | Coded Level | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | |
| Experimental Actual Values | |||||
| Extraction time (X1) | 11.6 | 15 | 20 | 25 | 28.4 |
| Temperature (X2) | 33.2 | 40 | 50 | 60 | 66.8 |
| Acetic acid Conc. (X3) | 33.2 | 40 | 50 | 60 | 66.8 |
| Runs | Time (X1) (min) | Temperature (X2) (°C) | Solvent Concentration (X3) (%) | Yield (g/100 g) | TPC mg GAE/g | TFC (mg QEQ/g) | DPPH (%) |
|---|---|---|---|---|---|---|---|
| 1 | 0 (20) | 0 (50) | 0 (50) | 32.05 | 22.92 | 10.18 | 8.72 |
| 2 | 1 (25) | 1 (60) | 1 (60) | 30.475 | 34.39 | 11.84 | 9.404 |
| 3 | −1 (15) | −1 (40) | 1 (60) | 35.2 | 20.55 | 14.15 | 2.35 |
| 4 | −1 (15) | 1 (60) | −1 (40) | 33.35 | 19.12 | 4.76 | 0.73 |
| 5 | 0 (20) | 0 (50) | 0 (50) | 34.625 | 25.64 | 9.87 | 8.03 |
| 6 | 1 (25) | 1 (60) | −1 (40) | 28.1 | 25.78 | 12.98 | 6.5 |
| 7 | 0 (20) | 1.68 (67) | 0 (50) | 25.7 | 24.79 | 11.21 | 7.64 |
| 8 | −1 (15) | −1 (40) | −1 (40) | 38.175 | 25.32 | 9.51 | 1.79 |
| 9 | 0 (20) | 0 (50) | 0 (50) | 33.75 | 24.92 | 12.54 | 8.24 |
| 10 | 1.68 (28) | 0 (50) | 0 (50) | 40 | 21.37 | 4.76 | 6.85 |
| 11 | −1.68 (12) | 0 (50) | 0 (50) | 32.4 | 19.92 | 8.9 | 3.45 |
| 12 | 0 (20) | 0 (50) | 0 (50) | 34.55 | 23.87 | 12.03 | 8.83 |
| 13 | 0 (20) | 0 (50) | −1.68 (33) | 38.775 | 19.63 | 5.32 | 3.32 |
| 14 | 0 (20) | −1.68 (33) | 0 (50) | 34.675 | 20.31 | 6.15 | 2.54 |
| 15 | 0 (20) | 0 (50) | 1.68 (67) | 37.25 | 20.19 | 10.26 | 7.68 |
| 16 | 1 (25) | −1 (40) | 1 (60) | 43 | 21.896 | 6.01 | 3.94 |
| 17 | −1 (15) | 1 (60) | −1 (40) | 30.85 | 12.77 | 9.65 | 4.93 |
| 18 | 1 (25) | −1 (40) | −1 (40) | 39.5 | 16.62 | 4.06 | 3.58 |
| Model Term | Extract Yield | TPC | TFC | Antioxidant Activity |
|---|---|---|---|---|
| Model (p-value) | 0.0011 | 0.0017 | 0.005 | 0.001 |
| Lack of Fit (p-value) | 0.22 | 0.17 | 0.37 | 0.07 |
| R2 | 0.92 | 0.91 | 0.88 | 0.95 |
| Source | df | Mean Squares | |||
|---|---|---|---|---|---|
| Yield | TPC | TFC | Antioxidant Activity | ||
| Model | 9 | 33.76 *** | 36.06 *** | 17.01 *** | 14.23 *** |
| X1-Time (Min) | 1 | 19.41 * | 39.98 ** | 7.51 * | 27.38 ** |
| X2-Temperature (°C) | 1 | 170.07 *** | 16.95 * | 14.36 ** | 25.03 ** |
| X3-Conc. (%) | 1 | 7.4 * | 22.25 * | 25.47 ** | 17.24 ** |
| X1X2 | 1 | 27.20 ** | 158.8 * | 72.0 *** | 5.88 * |
| X1X3 | 1 | 16.10 * | 78.17 *** | 9.51 * | 0.27 NS |
| X2X3 | 1 | 0.05 NS | 0.38 NS | 1.0 NS | 4.79 * |
| X12 | 1 | 9.38 * | 13.74 * | 17.96 ** | 23.53 ** |
| X22 | 1 | 20.22 ** | 1.72 NS | 3.67 NS | 24.23 ** |
| X32 | 1 | 28.54 ** | 21.42 * | 9.20 * | 19.45 ** |
| Residual | 8 | 2.95 | 3.55 | 2.39 | 0.66 |
| Lack of Fit | 5 | 3.86 NS | 4.83 NS | 2.76 NS | 0.97 NS |
| Pure Error | 3 | 1.43 | 1.41 | 1.77 | 0.14 |
| Optimized Condition | Coded Levels | Real Values |
| Time (min) | 1.3 | 26.5 |
| Temperature (°C) | 0.9 | 59 |
| Solvent Concentration (%) | 1.29 | 62.9 |
| Response | Experimental Values | Predicted Values |
| Yield (g/100 g) | 33.84 ± 1.8 | 34.95 |
| TPC mg (GAE/g) | 35.12 ± 2.3 | 34.38 |
| TFC (mg QEQ/g) | 10.05 ± 0.76 | 10.21 |
| Antioxidant activity mg/100 mL | 8.78 ± 0.91 | 9.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaleem, M.; Ahmad, A.; Amir, R.M.; Kaukab Raja, G. Ultrasound-Assisted Phytochemical Extraction Condition Optimization Using Response Surface Methodology from Perlette Grapes (Vitis vinifera). Processes 2019, 7, 749. https://doi.org/10.3390/pr7100749
Kaleem M, Ahmad A, Amir RM, Kaukab Raja G. Ultrasound-Assisted Phytochemical Extraction Condition Optimization Using Response Surface Methodology from Perlette Grapes (Vitis vinifera). Processes. 2019; 7(10):749. https://doi.org/10.3390/pr7100749
Chicago/Turabian StyleKaleem, Muhammad, Asif Ahmad, Rai Muhammad Amir, and Ghazala Kaukab Raja. 2019. "Ultrasound-Assisted Phytochemical Extraction Condition Optimization Using Response Surface Methodology from Perlette Grapes (Vitis vinifera)" Processes 7, no. 10: 749. https://doi.org/10.3390/pr7100749
APA StyleKaleem, M., Ahmad, A., Amir, R. M., & Kaukab Raja, G. (2019). Ultrasound-Assisted Phytochemical Extraction Condition Optimization Using Response Surface Methodology from Perlette Grapes (Vitis vinifera). Processes, 7(10), 749. https://doi.org/10.3390/pr7100749