Biogas Purification: A Comparison of Adsorption Performance in D4 Siloxane Removal Between Commercial Activated Carbons and Waste Wood-Derived Char Using Isotherm Equations
Abstract
:1. Introduction
2. Experimental Methods
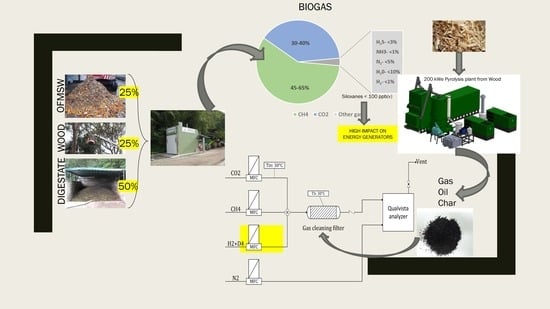
2.1. Description of the Experimental Setup
2.2. Methodology
2.3. Sorbent Characterization: SEM/EDS
3. Results and Discussion
3.1. Adsorption Capacity Related to Physical Sorbent Characteristics
3.2. Adsorption Isotherms and Experimental Modelling
3.2.1. Langmuir Isotherm
3.2.2. Freundlich Isotherm
3.2.3. Dubinin-Radushkevich Isotherm
3.2.4. Temkin Isotherm
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Cin | Inlet trace compound concentration (ppm(v)) |
| D3 | Hexamethylcyclotrisiloxane |
| D4 | Octamethylcyclotetrasiloxane |
| D5 | Decamethylcyclopentasiloxane |
| DFT | Density functional theory |
| EDS | Energy dispersive x-ray spectrometry |
| GHSV | Gas hourly space velocity (h−1) |
| Id | Internal diameter |
| IR | Infrared light source |
| L/D | Length/Diameter ratio |
| L4 | Decamethyltetrasiloxane |
| M | Mass of sorbent (g) |
| MFC | Mass Flow Controller |
| MW | Molecular weight of the trace compound removed (g/mol) |
| NDIR | Nondispersive infrared sensor |
| NLDFT | Non-localized density functional theory |
| OFMSW | Organic fraction of municipal solid waste |
| PDMS | Polymeric dimethylsiloxane membrane |
| PFA | Perfluoroalkoxy alkane polymers |
| ppb(v) | Parts per billion by volume |
| ppm(v) | Parts per million by volume |
| qmeasured | Amount od siloxane adsorbed (mg/g) |
| Qtot | Total gas flow rate (N·L/h) |
| RH | Relative Humidity |
| SEM | Scanning electron microscopy |
| SOFC | Solid oxide fuel cell |
| SSA | Specific surface area (m2/g) |
| STP | Standard Temperature and Pressure |
| Tb | Bulk Temperature of gas cleaning filter |
| Vm | Molar volume (22.414 N·L/mol) |
| Vmeso | Mesoporous volume (cm3/g): 2–50 nm |
| Vmicro | Microporous volume (cm3/g): <2 nm |
| VOCs | Volatile organic compounds |
References
- Papurello, D.; Soukoulis, C.; Schuhfried, E.; Cappellin, L.; Gasperi, F.; Silvestri, S.; Santarelli, M.; Biasioli, F. Monitoring of volatile compound emissions during dry anaerobic digestion of the Organic Fraction of Municipal Solid Waste by Proton Transfer Reaction Time-of-Flight Mass Spectrometry. Bioresour. Technol. 2012, 126, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M. Reduction and monitoring of biogas trace compounds. VTT Tied. Valt. Tek. Tutk. 2009, 2496, 27. [Google Scholar]
- Choudhury, A.; Chandra, H.; Arora, A. Application of solid oxide fuel cell technology for power generation—A review. Renew. Sustain. Energy Rev. 2013, 20, 430–442. [Google Scholar] [CrossRef]
- Lanzini, A.; Madi, H.; Chiodo, V.; Papurello, D.; Maisano, S.; Santarelli, M. Dealing with fuel contaminants in biogas-fed solid oxide fuel cell (SOFC) and molten carbonate fuel cell (MCFC) plants: Degradation of catalytic and electro-catalytic active surfaces and related gas puri fi cation methods. Prog. Energy Combust. Sci. 2017, 61, 150–188. [Google Scholar] [CrossRef]
- Sasaki, K.; Haga, K.; Yoshizumi, T.; Minematsu, D.; Yuki, E.; Liu, R.; Uryu, C.; Oshima, T.; Ogura, T.; Shiratori, Y.; et al. Chemical durability of Solid Oxide Fuel Cells: Influence of impurities on long-term performance. J. Power Sour. 2011, 196, 9130–9140. [Google Scholar] [CrossRef]
- Papurello, D.; Iafrate, C.; Lanzini, A.; Santarelli, M. Trace compounds impact on SOFC performance: Experimental and modelling approach. Appl. Energy 2017, 208, 637–654. [Google Scholar] [CrossRef]
- Papurello, D.; Lanzini, A. SOFC single cells fed by biogas: Experimental tests with trace contaminants. Waste Manag. 2018, 72, 306–312. [Google Scholar] [CrossRef]
- Madi, H.; Lanzini, A.; Papurello, D.; Diethelm, S.; Ludwig, C.; Santarelli, M. Solid oxide fuel cell anode degradation by the effect of hydrogen chloride in stack and single cell environments. J. Power Sour. 2016, 326, 349–356. [Google Scholar] [CrossRef]
- Madi, H.; Lanzini, A.; Diethelm, S.; Papurello, D.; Lualdi, M.; Larsen, J.G.; Santarelli, M. Solid oxide fuel cell anode degradation by the effect of siloxanes. J. Power Sour. 2015, 279, 460–471. [Google Scholar] [CrossRef]
- Haga, K.; Adachi, S.; Shiratori, Y.; Itoh, K.; Sasaki, K. Poisoning of SOFC anodes by various fuel impurities. Solid State Ion. 2008, 179, 1427–1431. [Google Scholar] [CrossRef]
- Subramanian, S.; Pande, G.; De Weireld, G.; Giraudon, J.M.; Lamonier, J.F.; Batra, V.S. Sugarcane bagasse fly ash as an attractive agro-industry source for VOC removal on porous carbon. J. Hazard. Mater. 2013, 49, 683–690. [Google Scholar] [CrossRef]
- Papurello, D.; Borchiellini, R.; Bareschino, P.; Chiodo, V.; Freni, S.; Lanzini, A.; Pepe, F.; Ortigoza, G.A.; Santarelli, M. Performance of a Solid Oxide Fuel Cell short-stack with biogas feeding. Appl. Energy 2014, 125, 254–263. [Google Scholar] [CrossRef]
- Papurello, D.; Lanzini, A.; Leone, P.; Santarelli, M.; Silvestri, S. Biogas from the organic fraction of municipal solid waste: Dealing with contaminants for a solid oxide fuel cell energy generator. Waste Manag. 2014, 34, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Hagen, A.; Rasmussen, J.F.B.; Thydén, K. Durability of solid oxide fuel cells using sulfur containing fuels. J. Power Sour. 2011, 196, 7271–7276. [Google Scholar] [CrossRef]
- Nair, N.; Zhang, X.; Gutierrez, J.; Chen, J.; Egolfopoulos, F.; Tsotsis, T. Impact of Siloxane Impurities on the Performance of an Engine Operating on Renewable Natural Gas. Ind. Eng. Chem. Res. 2012, 51, 15786–15795. [Google Scholar] [CrossRef]
- Jalali, A.; Motamedhashemi, M.M.Y.; Egolfopoulos, F.N.; Tsotsis, T. Fate of Siloxane Impurities During the Combustion of Renewable Natural Gas. Combust. Sci. Technol. 2013, 185, 953–974. [Google Scholar] [CrossRef]
- Madi, H.; Diethelm, S.; Poitel, S.; Ludwig, C.; Van Herle, J. Damage of Siloxanes on Ni-YSZ Anode Supported SOFC Operated on Hydrogen and Bio-Syngas. Fuel Cells 2015, 15, 718–727. [Google Scholar] [CrossRef]
- Abatzoglou, N.; Boivin, S. A review of biogas purification processes. Biofuels Bioprod. Biorefining 2009, 6, 42–71. [Google Scholar] [CrossRef]
- Dewil, R.; Appels, L.; Baeyens, J. Energy use of biogas hampered by the presence of siloxanes. Energy Convers. Manag. 2006, 47, 1711–1722. [Google Scholar] [CrossRef]
- Papurello, D.; Silvestri, S.; Tomasi, L.; Belcari, I.; Biasioli, F.; Santarelli, M. Biowaste for SOFCs. Energy Procedia 2016, 101, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Gong, H.; Chen, Z.; Zhang, M. Adsorption characteristics of activated carbon for siloxanes. Biochem. Pharmacol. 2013, 1, 1182–1187. [Google Scholar] [CrossRef]
- Papurello, D.; Schuhfried, E.; Lanzini, A.; Romano, A.; Cappellin, L.; Märk, T.D.; Silvestri, S.; Santarelli, M.; Biasioli, F. Proton transfer reaction-mass spectrometry as a rapid inline tool for filter efficiency of activated charcoal in support of the development of Solid Oxide Fuel Cells fueled with biogas. Fuel Process. Technol. 2015, 130, 78–86. [Google Scholar] [CrossRef]
- Papurello, D.; Tognana, L.; Lanzini, A.; Smeacetto, F.; Santarelli, M.; Belcari, I.; Silvestri, S.; Biasioli, F. Proton transfer reaction mass spectrometry technique for the monitoring of volatile sulfur compounds in a fuel cell quality clean-up system. Fuel Process. Technol. 2015, 130, 136–146. [Google Scholar] [CrossRef]
- Kupecki, J.; Kluczowski, R.; Papurello, D.; Lanzini, A.; Kawalec, M.; Krauz, M.; Santarelli, M. Characterization of a circular 80 mm anode supported solid oxide fuel cell (AS-SOFC) with anode support produced using high-pressure injection molding (HPIM). Int. J. Hydrogen Energy 2018, 44, 19405–19411. [Google Scholar] [CrossRef]
- Papurello, D.; Tomasi, L.; Silvestri, S.; Santarelli, M. Evaluation of the Wheeler-Jonas parameters for biogas trace compounds removal with activated carbons. Fuel Process. Technol. 2016, 152, 93–101. [Google Scholar] [CrossRef]
- Finocchio, E.; Montanari, T.; Garuti, G.; Pistarino, C.; Federici, F.; Cugino, M.; Busca, G. Purification of biogases from siloxanes by adsorption: On the regenerability of activated carbon sorbents. Energy Fuels 2009, 23, 4156–4159. [Google Scholar] [CrossRef]
- Beese, J. Betriebsoptimierung der moto rischen Gasverwertung durch den Einsatz von Gasreinigungsanlagen; Siloxa Engineering AG. In Betriebsoptimierung der Moto Rischen Gasverwertung Durch den EINSATZ von Gasreinigungsanlagen; Siloxa Engineering AG, 2007. [Google Scholar]
- Lunghi, P.; Bove, R.; Desideri, U. Life-cycle-assessment of fuel-cells-based landfill-gas energy conversion technologies. J. Power Sour. 2004, 131, 120–126. [Google Scholar] [CrossRef]
- Arnold, M.; Kajolinna, T. Development of on-line measurement techniques for siloxanes and other trace compounds in biogas. Waste Manag. 2010, 30, 1011–1017. [Google Scholar] [CrossRef]
- Berger, A.H.; Bhown, A.S. Comparing Physisorption and Chemisorption Solid Sorbents for use Separating CO2 from Flue Gas using Temperature Swing Adsorption. Energy Procedia 2011, 4, 562–567. [Google Scholar] [CrossRef]
- Chaemchuen, S.; Zhou, K.; Verpoort, F. From Biogas to Biofuel: Materials Used for Biogas Cleaning to Biomethane. ChemBioEng Rev. 2016, 3, 250–265. [Google Scholar] [CrossRef]
- Nam, S.; Namkoong, W.; Kang, J.H.; Park, J.K.; Lee, N. Adsorption characteristics of siloxanes in landfill gas by the adsorption equilibrium test. Waste Manag. 2013, 33, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Elwell, A.C.; Elsayed, N.H.; Kuhn, J.N.; Joseph, B. Design and analysis of siloxanes removal by adsorption from landfill gas for waste-to-energy processes. Waste Manag. 2017, 73, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Codony, A.; Montes-Moran, M.A.; Sanchez-Polo, M.; Martin, M.J.; Gonzalez-Olmos, R. Biogas upgrading: Optimal activated carbon properties for siloxane removal. Environ. Sci. Technol. 2014, 48, 7187–7195. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Gutiérrez, N.; García, S.; Gil, M.V.; Rubiera, F.; Pevida, C. Towards bio-upgrading of biogas: Biomass waste-based adsorbents. Energy Procedia 2014, 63, 6527–6533. [Google Scholar] [CrossRef]
- Hao, W.; Björkman, E.; Lilliestråle, M.; Hedin, N. Activated carbons prepared from hydrothermally carbonized waste biomass used as adsorbents for CO2. Appl. Energy 2013, 112, 526–532. [Google Scholar] [CrossRef]
- Papurello, D.; Boschetti, A.; Silvestri, S.; Khomenko, I.; Biasioli, F. Real-time monitoring of removal of trace compounds with PTR-MS: Biochar experimental investigation. Renew. Energy 2018, 125, 344–355. [Google Scholar] [CrossRef]
- Rogers, K.A. Adsorption on activated carbon by hydrogen, methane and carbon dioxide gases and their mixtures at 212.deg.K to 301.deg.K and up tp 35 atmospheres. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 1973. [Google Scholar]
- Gandiglio, M.; Lanzini, A.; Santarelli, M.; Acri, M.; Hakala, T.; Rautanen, M. Results from an industrial size biogas-fed SOFC plant (the DEMOSOFC project). Int. J. Hydrogen Energy 2019, in press. [Google Scholar] [CrossRef]
- Santarelli, M. NEWS FEATURE DEMOSOFC project to install first European plant to produce clean energy from The new DEMOSOFC project is focused on the installation of the first. Fuel Cells Bull. 2015, 2015, 14–15. [Google Scholar] [CrossRef]
- Beeckman, J.W.L.; Fassbender, N.A.; Datz, T.E. Length to Diameter Ratio of Extrudates in Catalyst Technology I. Modeling Catalyst Breakage by Impulsive Forces. AIChE J. 2016, 62, 639–647. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Hernàndez-Balada, E.; Mata-Álvarez, J.; Sisani, E. Performance characterization of a novel Fe-based sorbent for anaerobic gas desulfurization finalized to high temperature fuel cell applications. Int. J. Hydrogen Energy 2016, 42, 1859–1874. [Google Scholar] [CrossRef]
- Cui, H.; Turn, S.Q.; Reese, M.A. Removal of sulfur compounds from utility pipelined synthetic natural gas using modified activated carbons. Catal. Today 2009, 139, 274–279. [Google Scholar] [CrossRef]
- Qualvista. Qualvista Biogas Monitor. Qualvista. Ltd. Available online: http://www.qualvista.com/product-information-2/ (accessed on 12 September 2019).
- Jagiello, J.; Kenvin, J.; Oliver, J.; Lupini, A.; Contescu, C. Using a New Finite Slit Pore Model for NLDFT Analysis of Carbon Pore Structure. Adsorpt. Sci. Technol. 2011, 29, 769–780. [Google Scholar] [CrossRef]
- Tepper, F. Alkali metal, Chemical element. Encyclopædia Britannica Online. Available online: http://www.britannica.com/science/alkali-metal (accessed on 3 September 2015).
- Richardson, J.; Björheden, R.; Hakkila, P.; Lowe, A.T.; Smith, C.T. Bioenergy from Sustainable Forestry—Guiding Principles and Practice; Kluwer Academic Publisher: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 2002. [Google Scholar]
- Oshita, K.; Ishihara, Y.; Takaoka, M.; Takeda, N.; Matsumoto, T.; Morisawa, S.; Kitayama, A. Behaviour and adsorptive removal of siloxanes in sewage sludge biogas. Water Sci. Technol. 2010, 61, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Do, D.D. Adsorption Analysis: Equilibria and Kinetics; Imperial College Press: London, UK, 1998; Volume 2. [Google Scholar]
- Keller, J.U. Fundamentals of Adsorption; Kluwer Aca: Boston, MA, USA, 1996. [Google Scholar]
- Vijayaraghavan, K.; Padmesh, T.V.N.; Palanivelu, K.; Velan, M. Biosorption of nickel (II) ions onto Sargassum wightii: Application of two-parameter and three-parameter isotherm models. J. Hazard. Mater. 2006, 133, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Adamson, A.W.; Gast, A.P. Physical Chemistry Of Surfaces, 6th ed.; Wiley, Interscience Publishers, Inc.: New York, NY, USA, 1997. [Google Scholar]
- Dubinin, M.M.; Radushkevich, L.V. The equation of the characteristic curve of the activated charcoal. Proc. Acad. Sci. USSR Phys. Chem. 1947, 55, 331–337. [Google Scholar]
- Temkin, M.I.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys. Chim. USSR 1940, 12, 327–356. [Google Scholar]






| Test | S-01 | S-02 | S-05 | S-10 |
|---|---|---|---|---|
| CO2 (N·mL/min) | 175 | 175 | 175 | 175 |
| CH4 (N·mL/min) | 300 | 275 | 200 | 75 |
| H2 + D4 (N·mL/min) | 25 | 50 | 125 | 250 |
| Total flow rate (N·mL/min) | 500 | 500 | 500 | 500 |
| CH4 (%) | 60 | 55 | 40 | 15 |
| CO2 (%) | 35 | 35 | 35 | 35 |
| D4 concentrate (ppm(v)) | 1.0 | 2.0 | 5.0 | 10.0 |
| D4 (mg/m3) | 4.6 | 9.2 | 23.0 | 45.9 |
| Element | Biochar | C64 (Airdep, Verona, Italy) | CKC (Airdep, Verona, Italy) |
|---|---|---|---|
| C | 99.5 | 81.89 | 80.83 |
| O | - | 13.47 | 14.04 |
| Si | - | 0.99 | 1.03 |
| Al | - | 0.80 | 0.79 |
| K | 0.24 | 0.74 | 1.05 |
| Ca | 0.28 | 0.76 | 0.89 |
| Fe | - | 0.74 | 0.81 |
| S | - | 0.30 | 0.38 |
| Mg | - | 0.17 | 0.16 |
| Na | - | 0.14 | - |
| Variable | Unit | Biochar | C64 (Airdep, Italy) | CKC (Airdep, Italy) |
|---|---|---|---|---|
| Specific surface area | (m2/g) | 75.3 | 796.8 | 663.5 |
| V microporous (<2 nm) | (cm3/g) | 0.02 | 0.29 | 0.22 |
| V mesoporous (2–45 nm) | (cm3/g) | 0.02 | 0.066 | 0.187 |
| Total pore volume | (cm3/g) | 0.04 | 0.358 | 0.408 |
| Si (mg/m3) | qmeasured (mg/g) | Langmuir qcalculated (mg/g) | Error (%) | Freundlich qcalculated (mg/g) | Error (%) | DR qcalculated (mg/g) | Error (%) | Temkin qcalculated (mg/g) | Error (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Biochar | 2.3 | 2.64 | 2.83 | 6.89 | 2.84 | 7.34 | 2.64 | 0.12 | 2.85 | 7.5 |
| 4.59 | 3.5 | 3.53 | 0.79 | 3.3 | 5.86 | 3.5 | 0.9 | 3.38 | 3.73 | |
| 9.19 | 4.2 | 4.03 | 4.77 | 3.85 | 9.64 | 4.1 | 3.17 | 3.9 | 8.1 | |
| 22.97 | 4.37 | 4.4 | 0.64 | 4.7 | 6.98 | 4.47 | 2.08 | 4.60 | 4.96 | |
| C64 | 4.59 | 37.28 | 39.03 | 4.47 | 39.08 | 4.59 | 37.5 | 0.6 | 39.13 | 4.7 |
| 9.19 | 44.93 | 44.62 | 0.7 | 42.63 | 5.4 | 44.4 | 1.19 | 42.94 | 4.63 | |
| 22.97 | 49.5 | 48.82 | 1.4 | 47.83 | 3.49 | 49.13 | 0.75 | 47.99 | 3.15 | |
| 45.94 | 50.15 | 50.4 | 0.48 | 52.18 | 3.88 | 50.82 | 1.3 | 51.8 | 3.19 | |
| CKC | 4.59 | 25.17 | 27.12 | 7.17 | 26.88 | 6.36 | 25.4 | 0.97 | 26.97 | 6.65 |
| 9.19 | 32.4 | 32 | 1.2 | 30.16 | 7.4 | 31.82 | 1.8 | 30.56 | 6 | |
| 22.97 | 37 | 35.90 | 3.07 | 35.1 | 5.37 | 36.4 | 1.6 | 35.32 | 4.77 | |
| 45.94 | 37.18 | 37.4 | 0.6 | 39.40 | 5.63 | 38.09 | 2.39 | 38.9 | 4.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papurello, D.; Gandiglio, M.; Kafashan, J.; Lanzini, A. Biogas Purification: A Comparison of Adsorption Performance in D4 Siloxane Removal Between Commercial Activated Carbons and Waste Wood-Derived Char Using Isotherm Equations. Processes 2019, 7, 774. https://doi.org/10.3390/pr7100774
Papurello D, Gandiglio M, Kafashan J, Lanzini A. Biogas Purification: A Comparison of Adsorption Performance in D4 Siloxane Removal Between Commercial Activated Carbons and Waste Wood-Derived Char Using Isotherm Equations. Processes. 2019; 7(10):774. https://doi.org/10.3390/pr7100774
Chicago/Turabian StylePapurello, Davide, Marta Gandiglio, Jalal Kafashan, and Andrea Lanzini. 2019. "Biogas Purification: A Comparison of Adsorption Performance in D4 Siloxane Removal Between Commercial Activated Carbons and Waste Wood-Derived Char Using Isotherm Equations" Processes 7, no. 10: 774. https://doi.org/10.3390/pr7100774
APA StylePapurello, D., Gandiglio, M., Kafashan, J., & Lanzini, A. (2019). Biogas Purification: A Comparison of Adsorption Performance in D4 Siloxane Removal Between Commercial Activated Carbons and Waste Wood-Derived Char Using Isotherm Equations. Processes, 7(10), 774. https://doi.org/10.3390/pr7100774