Assessment of the Total Volume Membrane Charge Density through Mathematical Modeling for Separation of Succinic Acid Aqueous Solutions on Ceramic Nanofiltration Membrane
Abstract
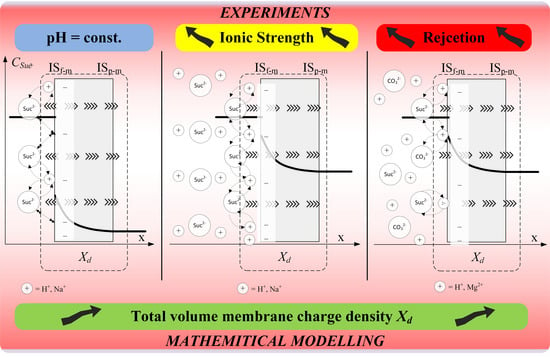
:1. Introduction
2. Experimental Methods
2.1. Experimental and Operating Conditions
2.2. Materials
2.3. Analysis
3. Modeling of Nanofiltration
3.1. Theory of the Utilized Model
3.2. Determination of Total Volume Membrane Charge Density Values in Nanofiltration
4. Results and Discussion
- Solute parameters: molecular size, acid dissociation constant, hydrophobicity/hydrophilicity, and diffusion coefficient,
- Membrane properties: cut-off, pore size, surface charge,
- Feed composition: pH, ionic strength, hardness, and the presence of organic matter.
4.1. Impact of Dynamic Viscosity on Modeled Permeate Flux
4.2. Comparison of the ddDSPM Model with the Standard Approach
4.3. Variation of the Overall Volume Charge Densities in Relation to Used pH Regulator
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Ak | mean membrane porosity, % |
| Cf,H2O | water molar concentration in feed, mol/m3 |
| Cf,i | ion concentration in the feed, mol/m3 |
| Cp.i | ion concentration in the permeate, mol/m3 |
| Di | diffusion coefficient of component or ion, m2/s |
| F | Faraday constant, C/mol |
| I | ionic strength, mol/dm3 |
| ISf-m | feed-membrane interface |
| ISf-m | permeate-membrane interface |
| Kc,I | hindrance factor for convection |
| Kd,I | hindrance factor for diffusion |
| MS | model solution, numbering and details according the Table 1 |
| N | total number of measurements taken during all the experiments |
| NC | number of components |
| NE | number of experiments performed |
| NMij | number of measurements of the j-th variable in the i-th experiment |
| NVi | number of variables measured in the i-th experiment |
| R | ideal gas constant, J/(mol × K) |
| Ri | retention coefficient of component i |
| T | temperature, K |
| V | solvent velocity (volume flux), m3/(m2 × s) |
| Ṽw | molar volume of water, m3/mol |
| Xd | effective membrane charge density, mol/m3 |
| cijk | k-th predicted value of variable j in experiment i |
| cijk,mes | k-th measured value of variable j in experiment i |
| cm(0+),i | ion concentration in the membrane in the surface directly contacting with the feed, mol/m3 |
| cm,i | concentration of ion in the membrane, mol/m3 |
| kB | Boltzmann constant (1.38×10−23 J/K) |
| rp | pore radii, m |
| rs,i | ion radii, m |
| xf,i | molar fraction on the feed side, mol/mol |
| xp,i | molar fraction on the permeate side, mol/mol |
| zi | charge of individual ion |
| Greek Letters | |
| θ | set of model parameters to be estimated |
| Ψ | potential gradient inside membrane pore, V |
| ΨD | Donnan potential, V |
| Δπ | osmotic pressure difference, Pa |
| ΔP | transmembrane pressure, Pa |
| Δx | thickness of membrane active layer, m |
| η | viscosity, Pa × s |
| ηs | solvent viscosity, Pa × s |
| λi | ratio of solute to pore radius |
| πpermeate | osmotic pressure on the permeate side, Pa |
| πfeed | osmotic pressure on the feed side, Pa |
| variance of the k-th measurement of variable j in experiment i | |
| Φi | steric term |
References
- Lisiecki, P.; Chrzanowski, Ł.; Szulc, A.; Ławniczak, Ł.; Białas, W.; Dziadas, M.; Owsianiak, M.; Staniewski, J.; Cyplik, P.; Marecik, R.; et al. Biodegradation of diesel/biodiesel blends in saturated sand microcosms. Fuel 2014, 116, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Chrzanowski, Ł.; Dziadas, M.; Ławniczak, Ł.; Cyplik, P.; Białas, W.; Szulc, A.; Lisiecki, P.; Jeleń, H. Biodegradation of rhamnolipids in liquid cultures: Effect of biosurfactant dissipation on diesel fuel/B20 blend biodegradation efficiency and bacterial community composition. Bioresour. Technol. 2012, 111, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- BP Statistical Review of World Energy. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2018-full-report.pdf (accessed on 22 January 2019).
- Lalman, J.A.; Shewa, W.A.; Gallagher, J.; Ravella, S. Biofuels Production from Renewable Feedstocks. In Quality Living Through Chemurgy and Green Chemistry; Springer: Berlin/Heidelberg, Germany, 2016; pp. 193–220. ISBN 9783662537022. [Google Scholar]
- Gao, C.; Yang, X.; Wang, H.; Rivero, C.P.; Li, C.; Cui, Z.; Qi, Q.; Lin, C.S.K. Robust succinic acid production from crude glycerol using engineered Yarrowia lipolytica. Biotechnol. Biofuels 2016, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Antczak, J.; Regiec, J.; Prochaska, K. Separation and concentration of succinic adic from multicomponent aqueous solutions by nanofiltration technique. Pol. J. Chem. Technol. 2014, 16, 1–4. [Google Scholar] [CrossRef]
- Becker, J.; Lange, A.; Fabarius, J.; Wittmann, C. Top value platform chemicals: Bio-based production of organic acids. Curr. Opin. Biotechnol. 2015, 36, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, E.; Szewczyk, K.W.; Jaworska, M.M.; Konieczna-Mordas, E. Biotechnologiczne wytwarzanie kwasu bursztynowego. Inż. Ap. Chem. 2012, 51, 171–173. [Google Scholar]
- Vinoth Kumar, R.; Pakshirajan, K.; Pugazhenthi, G. Malic and succinic acid: potential C4 platform chemicals for polymer and biodegradable plastic production. In Platform Chemical Biorefinery: Future Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 159–179. ISBN 978-0-12-802980-0. [Google Scholar]
- Van Heerden, C.D.; Nicol, W. Continuous succinic acid fermentation by Actinobacillus succinogenes. Biochem. Eng. J. 2013, 73, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, M.; Matos, M.; Roca, C.; Reis, M.A.M. Succinic acid production from glycerol by Actinobacillus succinogenes using dimethylsulfoxide as electron acceptor. New Biotechnol. 2014, 31, 133–139. [Google Scholar] [CrossRef]
- Bradfield, M.F.A.; Nicol, W. Continuous succinic acid production by Actinobacillus succinogenes in a biofilm reactor: Steady-state metabolic flux variation. Biochem. Eng. J. 2014, 85, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pateraki, C.; Patsalou, M.; Vlysidis, A.; Kopsahelis, N.; Webb, C.; Koutinas, A.A.; Koutinas, M. Actinobacillus succinogenes: Advances on succinic acid production and prospects for development of integrated biorefineries. Biochem. Eng. J. 2016, 112, 285–303. [Google Scholar] [CrossRef]
- Staszak, K.; Woźniak, M.; Sottek, M.; Karaś, Z.; Prochaska, K. Removal of fumaric acid from simulated and real fermentation broth. J. Chem. Technol. Biotechnol. 2015, 90, 432–440. [Google Scholar] [CrossRef]
- Erickson, B.; Nelson; Winters, P. Perspective on opportunities in industrial biotechnology in renewable chemicals. Biotechnol. J. 2012, 7, 176–185. [Google Scholar] [CrossRef] [PubMed]
- López-Garzón, C.S.; Straathof, A.J.J. Recovery of carboxylic acids produced by fermentation. Biotechnol. Adv. 2014, 32, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Efe, Ç.; Pieterse, M.; van der Wielen, L.A.M.; Straathof, A.J.J. Separation of succinic acid from its salts on a high-silica zeolite bed. Chem. Eng. Process. Process Intensif. 2011, 50, 1143–1151. [Google Scholar] [CrossRef]
- Pérez-Cisneros, E.S.; Mena-Espino, X.; Rodríguez-López, V.; Sales-Cruz, M.; Viveros-García, T.; Lobo-Oehmichen, R. An integrated reactive distillation process for biodiesel production. Comput. Chem. Eng. 2016, 91, 233–246. [Google Scholar] [CrossRef]
- Zaman, N.K.; Rohani, R.; Abdul Shukor, M.H.; Mohamad, A.W. Purification of High Value Succinic Acid from Biomass Fermentation Broth via Nanofiltration. Indian J. Sci. Technol. 2016, 9, 95244. [Google Scholar] [CrossRef]
- Zaman, N.K.; Rohani, R.; Mohammad, A.W.; Isloor, A.M.; Jahim, J.M. Investigation of succinic acid recovery from aqueous solution and fermentation broth using polyimide nanofiltration membrane. J. Environ. Chem. Eng. 2017. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Liu, Y.; Ou, S.; Tan, Y.; Tang, S. Refining of biodiesel by ceramic membrane separation. Fuel Process. Technol. 2009, 90, 422–427. [Google Scholar] [CrossRef]
- Blumenschein, S.; Böcking, A.; Kätzel, U.; Postel, S.; Wessling, M. Rejection modeling of ceramic membranes in organic solvent nanofiltration. J. Memb. Sci. 2016, 510, 191–200. [Google Scholar] [CrossRef]
- Hilal, N.; Al-Zoubi, H.; Darwish, N.A.; Mohamma, A.W.; Abu Arabi, M. A comprehensive review of nanofiltration membranes: Treatment, pretreatment, modelling, and atomic force microscopy. Desalination 2004, 170, 281–308. [Google Scholar] [CrossRef]
- Bowen, W.R.; Welfoot, J.S. Predictive modelling of nanofiltration: Membrane specification and process optimisation. Desalination 2002, 147, 197–203. [Google Scholar] [CrossRef]
- Mitkowski, P.T.; Buchaly, C.; Kreis, P.; Jonsson, G.; Górak, A.; Gani, R. Computer aided design, analysis and experimental investigation of membrane assisted batch reaction-separation systems. Comput. Chem. Eng. 2009, 33, 551–574. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Mänttäri, M.; Nyström, M. Drawbacks of applying nanofiltration and how to avoid them: a review. Sep. Purif. Technol. 2008, 63, 251–263. [Google Scholar] [CrossRef]
- Vandezande, P.; Gevers, L.E.M.; Vankelecom, I.F.J. Solvent resistant nanofiltration: Separating on a molecular level. Chem. Soc. Rev. 2008, 37, 365–405. [Google Scholar] [CrossRef] [PubMed]
- Szymczyk, A.; Fatin-Rouge, N.; Fievet, P.; Ramseyer, C.; Vidonne, A. Identification of dielectric effects in nanofiltration of metallic salts. J. Memb. Sci. 2007, 287, 102–110. [Google Scholar] [CrossRef]
- Szymczyk, A.; Fievet, P. Investigating transport properties of nanofiltration membranes by means of a steric, electric and dielectric exclusion model. J. Memb. Sci. 2005, 252, 77–88. [Google Scholar] [CrossRef]
- Bellona, C.; Drewes, J.E.; Xu, P.; Amy, G. Factors affecting the rejection of organic solutes during NF/RO treatment—A literature review. Water Res. 2004, 38, 2795–2809. [Google Scholar] [CrossRef]
- Teixeira, M.; Rosa, M.; Nystrom, M. The role of membrane charge on nanofiltration performance. J. Memb. Sci. 2005, 265, 160–166. [Google Scholar] [CrossRef]
- Broniarz-Press, L.; Mitkowski, P.T.; Szaferski, W.; Marecka, A. Modelowanie procesu odzysku fumaranu diamonu metodą nanofiltracji. Inż. Ap. Chem. 2014, 53, 223–224. [Google Scholar]
- Mitkowski, P.T.; Broniarz-Press, L.; Marecka, A. Knowledge and model-based framework for nanofiltration modelling. In Proceedings of the 4th European Young Engineers Conference 2015, Warsaw, Poland, 27–29 April 2015. [Google Scholar]
- Perrin, D.D.; Dempsey, B.; Serjeant, E.P. pKa Prediction for Organic Acids and Bases; Springer: Dordrecht, The Netherlands, 1981; ISBN 978-94-009-5885-2. [Google Scholar]
- Puhlfürß, P.; Voigt, A.; Weber, R.; Morbé, M. Microporous TiO2 membranes with a cut off <500 Da. J. Memb. Sci. 2000, 174, 123–133. [Google Scholar]
- Zhang, Q.; Jing, W.; Fan, Y.; Xu, N. An improved Parks equation for prediction of surface charge properties of composite ceramic membranes. J. Memb. Sci. 2008, 318, 100–106. [Google Scholar] [CrossRef]
- Kong, F.; Yang, H.; Wang, X.; Xie, Y.F. Assessment of the hindered transport model in predicting the rejection of trace organic compounds by nanofiltration. J. Memb. Sci. 2016, 498, 57–66. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W. Mathematical modeling of membrane operations for water treatment. In Advances in Membrane Technologies for Water Treatment; Elsevier: Amsterdam, The Netherlands, 2015; pp. 379–407. ISBN 9781782421214. [Google Scholar]
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular separation with organic solvent nanofiltration: A critical review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef] [PubMed]
- Darvishmanesh, S.; Buekenhoudt, A.; Degrève, J.; Van der Bruggen, B. General model for prediction of solvent permeation through organic and inorganic solvent resistant nanofiltration membranes. J. Memb. Sci. 2009, 334, 43–49. [Google Scholar] [CrossRef]
- Tanimura, S.; Nakao, S.I.; Kimura, S. Transport equation for a membrane based on a frictional model. J. Memb. Sci. 1993, 84, 79–91. [Google Scholar] [CrossRef]
- Bowen, W.R.; Mukhtar, H. Characterisation and prediction of separation performance of nanofiltration membranes. J. Memb. Sci. 1996, 112, 263–274. [Google Scholar] [CrossRef]
- Bowen, W.R.; Mohammad, A.W. Diafiltration by nanofiltration: Prediction and optimization. AIChE J. 1998, 44, 1799–1812. [Google Scholar] [CrossRef]
- Bowen, W.R.; Mohammad, A.W. A theoretical basis for specifying nanofiltration membranes-dye/salt/water streams. Desalination 1998, 117, 257–264. [Google Scholar] [CrossRef]
- Van Gestel, T.; Vandecasteele, C.; Buekenhoudt, A.; Dotremont, C.; Luyten, J.; Leysen, R.; Van der Bruggen, B.; Maes, G. Salt retention in nanofiltration with multilayer ceramic TiO2 membranes. J. Memb. Sci. 2002, 209, 379–389. [Google Scholar] [CrossRef]
- Bernata, X.; Fortuny, A.; Stüber, F.; Bengoa, C.; Fabregat, A.; Font, J. Recovery of iron (III) from aqueous streams by ultrafiltration. Desalination 2008, 221, 413–418. [Google Scholar] [CrossRef]
- Izadpanah, A.A.; Javidnia, A. The ability of a nanofiltration membrane to remove hardness and ions from diluted seawater. Water 2012, 4, 283–294. [Google Scholar] [CrossRef]
- Ramadan, Y.; Pátzay, G.; Szabó, G.T. Transport of NaCl, MgSO4, MgCl2 and Na2SO4 across DL type nanofiltration membrane. Period. Polytech. Chem. Eng. 2010, 54, 81. [Google Scholar] [CrossRef]
- Prochaska, K.; Antczak, J.; Regel-Rosocka, M.; Szczygiełda, M. Removal of succinic acid from fermentation broth by multistage process (membrane separation and reactive extraction). Sep. Purif. Technol. 2018, 192, 360–368. [Google Scholar] [CrossRef]
- Antczak, J.; Szczygiełda, M.; Prochaska, K. Nanofiltration separation of succinic acid from post-fermentation broth: Impact of process conditions and fouling analysis. J. Ind. Eng. Chem. 2019, 77, 253–261. [Google Scholar] [CrossRef]
- Matusiewicz, H.; Ślachciński, M.; Hidalgo, M.; Canals, A. Evaluation of various nebulizers for use in microwave induced plasma optical emission spectrometry. J. Anal. At. Spectrom. 2007, 22, 1174. [Google Scholar] [CrossRef]
- Braeken, L.; Bettens, B.; Boussu, K.; Van der Meeren, P.; Cocquyt, J.; Vermant, J.; Van der Bruggen, B. Transport mechanisms of dissolved organic compounds in aqueous solution during nanofiltration. J. Memb. Sci. 2006, 279, 311–319. [Google Scholar] [CrossRef]
- Labbez, C.; Fievet, P.; Thomas, F.; Szymczyk, A.; Vidonne, A.; Foissy, A.; Pagetti, P. Evaluation of the “DSPM” model on a titania membrane: Measurements of charged and uncharged solute retention, electrokinetic charge, pore size, and water permeability. J. Colloid Interface Sci. 2003, 262, 200–211. [Google Scholar] [CrossRef]
- Ilie, O.; van Loosdrecht, M.C.M.; Picioreanu, C. Mathematical modelling of tooth demineralisation and pH profiles in dental plaque. J. Theor. Biol. 2012, 309, 159–175. [Google Scholar] [CrossRef]
- Periodic Table of Elements: Hydrogen—H. Available online: http://environmentalchemistry.com/yogi/periodic/H.html (accessed on May 22, 2018).
- Lide, D.R. CRC Handbook of Chemistry and Physics, 84th ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Atomic and Ionic Radii—Wired Chemist. Available online: http://www.wiredchemist.com/chemistry/data/atomic-and-ionic-radii (accessed on 22 May 2018).
- Li, Y.-H.; Gregory, S. Diffusion of ions in sea water and in deep-sea sediments. Geochem. Cosmochim. Acta 1974, 38, 703–714. [Google Scholar]
- Process Systems Enterprise Limited gPROMS. Model Builder Documentation 2014. Release 4.0.0-April 2014; Process Systems Enterprise: London, UK, 2014 20 April. [Google Scholar]
- Aydiner, C.; Kaya, Y.; Gönder, Z.B.; Vergili, I. Evaluation of membrane fouling and flux decline related with mass transport in nanofiltration of tartrazine solution. J. Chem. Technol. Biotechnol. 2010, 85, 1229–1240. [Google Scholar] [CrossRef]
- Kamo, N.; Kobatake, Y. Fixed charge density effective to membrane phenomena—Part III. Transference number of small ions. Kolloid-Z. Z. Polym. 1971, 249, 1069–1076. [Google Scholar] [CrossRef]
- Bandini, S.; Bruni, L. Transport Phenomena in Nanofiltration Membranes. In Comprehensive Membrane Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2010; Volume 2, pp. 67–89. ISBN 9780080932507. [Google Scholar]
- Hagmeyer, G.; Gimbel, R. Modelling the rejection of nanofiltration membranes using zeta potential measurements. Sep. Purif. Technol. 1999, 15, 19–30. [Google Scholar] [CrossRef]
- Takagi, R.; Larbot, A.; Cot, L.; Nakagaki, M. Effect of Al2O3 support on electrical properties of TiO2/Al2O3 membrane formed by sol–gel method. J. Memb. Sci. 2000, 177, 33–40. [Google Scholar] [CrossRef]







| Model Solution | Concentration of Succinic Acid (g/L) | Compound Used to Regulate pH | Amount of Added Compound Which Regulated pH (g/L) |
|---|---|---|---|
| MS1 | 3.6 | NaOH | 2.49 |
| MS2 | 36.0 | NaOH | 24.26 |
| MS3 | 3.6 | 4MgCO3 × Mg(OH)2 × 5H2O | 3.85 |
| Differential variables | Unit | Number | |
| Concentration of ion in the membrane | cm,i | mol/m3 | NC |
| Algebraic and implicit variables | Unit | Number | |
| Potential gradient inside the membrane pore | ψ | V | 1 |
| Ratio of solute to pore radius | λi | – | NC |
| Steric term | ϕi | – | NC |
| Hindrance factor for diffusion | Kd,i | – | NC |
| Hindrance factor for convection | Kc,i | – | NC |
| Ion concentration in the permeate | Cp.i | mol/m3 | NC |
| Retention coefficient | Ri | % | NC |
| Solvent velocity (volume flux) | V | m3/(m2·s) | 1 |
| Donnan potential | ψD | V | 1 |
| Osmotic pressure difference | Δπ | Pa | 1 |
| Osmotic pressure on the feed side | πeed | Pa | 1 |
| Osmotic pressure on the permeate side | πpermeate | Pa | 1 |
| Molar fraction on the feed side | xf,i | mol/mol | NC |
| Molar fraction on the permeate side | xp,i | mol/mol | NC |
| Ion concentration in the membrane in the surface directly contacting with the feed | cm(0+),i | mol/m3 | NC |
| Parameters and known variable | Unit | Number | |
| Effective membrane charge density | Xd | mol/m3 | 1 |
| Pore radii | rp | m | 1 |
| Ion radii | rs,i | m | NC |
| Transmembrane pressure | ΔP | Pa | 1 |
| Ideal gas constant | R | J/(mol·K) | 1 |
| Faraday constant | F | C/mol | 1 |
| Temperature | T | K | 1 |
| Solvent viscosity | ηs | Pa·s | 1 |
| Thickness of membrane active layer | Δx | m | 1 |
| Molar volume of water | Ṽw | m3/mol | 1 |
| Diffusion coefficient of ion | Di | m2/s | NC |
| Mean membrane porosity | Ak | % | 1 |
| Charge of individual ion | zi | – | NC |
| Ion concentration in the feed | Cf,i | mol/m3 | NC |
| Water molar concentration in feed | Cf,H2O | mol/m3 | 1 |
| Total number of variables: 17 + 14NC |
| Description of Equations | Equations | Number of Equations |
|---|---|---|
| Solvent velocity (volume flux) based on Hagen–Poiseuille-type relationship | (2) | 1 |
| Osmotic pressure difference across the membrane | (3) | 1 |
| Component molar fraction in feed | (4) | NC |
| Component molar fraction in permeate | (5) | NC |
| Osmotic pressure at the feed side | (6) | 1 |
| Osmotic pressure at the permeate side | (7) | 1 |
| Ratio of the solute radii to the pore radii | (8) | NC |
| Steric partitioning coefficient | (9) | NC |
| Hindrance factor for diffusion | (10) | NC |
| Hindrance factor for convection | (11) | NC |
| Concentration gradient inside the membrane pore | (12) | NC |
| Potential gradient inside the membrane pore | (13) | 1 |
| Electroneutrality conditions in the membrane | (14) | 1 |
| Electroneutrality conditions in the permeate | (15) | 1 |
| Donnan steric partitioning | (16) | NC |
| Retention coefficient | (17) | NC |
| Boundary condition at the membrane feed side | (18) | NC |
| Boundary condition at the membrane active layer thickness | (19) | NC |
| Total number of equations:7 + 9NC + 2NC (boundary conditions) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marecka-Migacz, A.; Tomasz Mitkowski, P.; Antczak, J.; Różański, J.; Prochaska, K. Assessment of the Total Volume Membrane Charge Density through Mathematical Modeling for Separation of Succinic Acid Aqueous Solutions on Ceramic Nanofiltration Membrane. Processes 2019, 7, 559. https://doi.org/10.3390/pr7090559
Marecka-Migacz A, Tomasz Mitkowski P, Antczak J, Różański J, Prochaska K. Assessment of the Total Volume Membrane Charge Density through Mathematical Modeling for Separation of Succinic Acid Aqueous Solutions on Ceramic Nanofiltration Membrane. Processes. 2019; 7(9):559. https://doi.org/10.3390/pr7090559
Chicago/Turabian StyleMarecka-Migacz, Agata, Piotr Tomasz Mitkowski, Jerzy Antczak, Jacek Różański, and Krystyna Prochaska. 2019. "Assessment of the Total Volume Membrane Charge Density through Mathematical Modeling for Separation of Succinic Acid Aqueous Solutions on Ceramic Nanofiltration Membrane" Processes 7, no. 9: 559. https://doi.org/10.3390/pr7090559
APA StyleMarecka-Migacz, A., Tomasz Mitkowski, P., Antczak, J., Różański, J., & Prochaska, K. (2019). Assessment of the Total Volume Membrane Charge Density through Mathematical Modeling for Separation of Succinic Acid Aqueous Solutions on Ceramic Nanofiltration Membrane. Processes, 7(9), 559. https://doi.org/10.3390/pr7090559