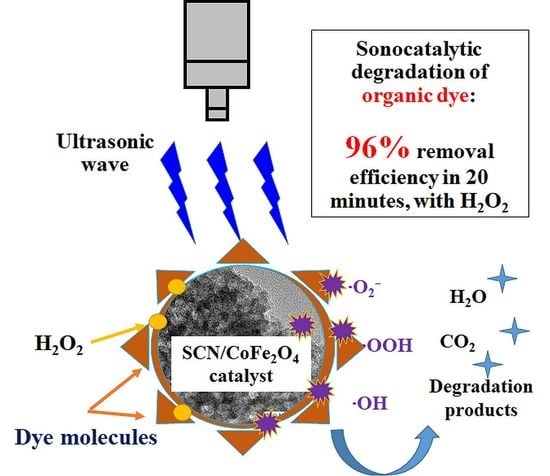
Ultrasonically Induced Sulfur-Doped Carbon Nitride/Cobalt Ferrite Nanocomposite for Efficient Sonocatalytic Removal of Organic Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of SCN
2.3. Ultrasonic Synthesis of SCN/CoFe2O4 Nanocomposite
2.3.1. Characterization
2.3.2. Sonocatalytic Degradation of Organic Dyes
3. Results and Discussion
3.1. Characterizations of the SCN, CoFe2O4 and SCN/CoFe2O4 Catalysts
3.2. Effect of Catalyst Dosage
3.3. Effect of H2O2 Loading
3.4. Sonocatalytic Degradation Performances of the SCN, CoFe2O4 and SCN/CoFe2O4 Catalysts
3.5. Effect of the Type of Organic Dye on Sonodegradation
3.6. Study of the Possible Mechanism of Sonodegradation
3.7. Reusability and Stability of SCN/CoFe2O4 Nanocomposite
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, S.; Tang, Y.; Xie, Y.; Tian, C.; Feng, Q.; Zhou, W.; Jiang, B. P-doped tubular g-C3N4 with surface carbon defects: Universal synthesis and enhanced visible-light photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 218, 664–671. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Zhang, Q.; Qin, P.; Wang, X.; He, G.; Li, L. One-step synthesis of WO3-CuS nanosheets heterojunction with enhanced photocatalytic performance for methylene blue degradation and Cr (VI) reduction. J. Chem. Technol. Biotechnol 2019. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.; Jiang, M.; Li, J.; Sheng, J. Removal of methylene blue from aqueous solution by adsorption on pyrophyllite. J. Mol. Liq. 2015, 209, 267–271. [Google Scholar] [CrossRef]
- Ertugay, N.; Acar, F.N. The degradation of Direct Blue 71 by sono, photo and sonophotocatalytic oxidation in the presence of ZnO nanocatalyst. Appl. Surf. Sci. 2014, 318, 121–126. [Google Scholar] [CrossRef]
- Hassani, A.; Eghbali, P.; Metin, Ö. Sonocatalytic removal of methylene blue from water solution by cobalt ferrite/mesoporous graphitic carbon nitride (CoFe2O4/mpg-C3N4) nanocomposites: Response surface methodology approach. Environ. Sci. Pollut. Res. 2018, 25, 32140–32155. [Google Scholar] [CrossRef]
- Hassani, A.; Çelikdağ, G.; Eghbali, P.; Sevim, M.; Karaca, S.; Metin, Ö. Heterogeneous sono-Fenton-like process using magnetic cobalt ferrite-reduced graphene oxide (CoFe2O4-rGO) nanocomposite for the removal of organic dyes from aqueous solution. Ultrason. Sonochem. 2018, 40, 841–852. [Google Scholar] [CrossRef]
- Sadhanala, H.K.; Maddegalla, A.; Nanda, K. Thioacetamide-derived nitrogen and sulfur co-doped carbon nanoparticles used for label-free detection of copper (ii) ions and bioimaging applications. New J. Chem. 2017, 41, 13742–13746. [Google Scholar] [CrossRef]
- Hu, S.; Yang, L.; Tian, Y.; Wei, X.; Ding, J.; Zhong, J.; Chu, P.K. Non-covalent doping of graphitic carbon nitride with ultrathin graphene oxide and molybdenum disulfide nanosheets: An effective binary heterojunction photocatalyst under visible light irradiation. J. Colloid Interface Sci. 2014, 431, 42–49. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, R.K.; Park, N.-J.; Baeg, J.-O. Facile one-pot two-step synthesis of novel in situ selenium-doped carbon nitride nanosheet photocatalysts for highly enhanced solar fuel production from CO2. ACS Appl. Nano Mater. 2017, 1, 47–54. [Google Scholar] [CrossRef]
- Feng, S.; Yan, P.; Xu, L.; Xia, J.; Li, H. Exploitation of a photoelectrochemical sensing platform for bisphenol A quantitative determination using Cu/graphitic carbon nitride nanocomposites. Chin. Chem. Lett. 2018, 29, 1629–1632. [Google Scholar] [CrossRef]
- Rivera-Tapia, E.D.; Fajardo, C.A.; Ávila-Vega, Á.J.; Ávila, C.F.; Sánchez-Arévalo, F.M.; Chango-Villacís, I.; Quiroz-Chávez, F.J.; Santoyo-Salazar, J.; Dante, R.C. Synthesis of boron carbon nitride oxide (BCNO) from urea and boric acid. Fuller. Nanotub. Carbon Nanostruct. 2016, 24, 8–12. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, X.; Chang, B.; Zhou, Y.; Zhang, S.; He, G.; Yang, B.; Li, J. Fabrication of B doped g-C3N4/TiO2 heterojunction for efficient photoelectrochemical water oxidation. Electrochim. Acta 2018, 282, 767–774. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Lv, W.; Zhu, B.; Tong, F.; Pan, J.; Bai, J.; Zhou, Q.; Qin, H. Template-Free One-Step Synthesis of g-C3N4 Nanosheets with Simultaneous Porous Network and S-Doping for Remarkable Visible-Light-Driven Hydrogen Evolution. ACS Sustain. Chem. Eng. 2019, 7, 5801–5807. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Y.; Liu, X.; Zeng, G.; Shao, B.; Liu, Y.; Liu, Y.; Zhang, W.; Yan, M.; He, X. Silver chromate modified sulfur doped graphitic carbon nitride microrod composites with enhanced visible-light photoactivity towards organic pollutants degradation. Compos. Part B Eng. 2019, 173, 106918. [Google Scholar] [CrossRef]
- Cao, S.; Huang, Q.; Zhu, B.; Yu, J. Trace-level phosphorus and sodium co-doping of g-C3N4 for enhanced photocatalytic H2 production. J. Power Sources 2017, 351, 151–159. [Google Scholar] [CrossRef]
- Liu, B.; Ye, L.; Wang, R.; Yang, J.; Zhang, Y.; Guan, R.; Tian, L.; Chen, X. Phosphorus-doped graphitic carbon nitride nanotubes with amino-rich surface for efficient CO2 capture, enhanced photocatalytic activity, and product selectivity. ACS Appl. Mater. Interfaces 2018, 10, 4001–4009. [Google Scholar] [CrossRef]
- Kumar, P.; Boukherroub, R.; Shankar, K. Sunlight-driven water-splitting using two-dimensional carbon based semiconductors. J. Mater. Chem. A 2018, 6, 12876–12931. [Google Scholar] [CrossRef]
- Nasir, M.S.; Yang, G.; Ayub, I.; Wang, S.; Wang, L.; Wang, X.; Yan, W.; Peng, S.; Ramakarishna, S. Recent development in graphitic carbon nitride based photocatalysis for hydrogen generation. Appl. Catal. B Environ. 2019, 117855. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, H.; Sun, H.; Liu, S.; Tade, M.O.; Wang, S.; Jin, W. Recent advances in non-metal modification of graphitic carbon nitride for photocatalysis: A historic review. Catal. Sci. Technol. 2016, 6, 7002–7023. [Google Scholar] [CrossRef]
- Zhang, Y.; Zong, S.; Cheng, C.; Shi, J.; Guan, X.; Lu, Y.; Guo, L. One-pot annealing preparation of Na-doped graphitic carbon nitride from melamine and organometallic sodium salt for enhanced photocatalytic H2 evolution. Int. J. Hydrog. Energy 2018, 43, 13953–13961. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A reveiw. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Shcherban, N.D.; Filonenko, S.M.; Ovcharov, M.L.; Mishura, A.M.; Skoryk, M.A.; Aho, A.; Murzin, D.Y. Simple method for preparing of sulfur–doped graphitic carbon nitride with superior activity in CO2 photoreduction. ChemistrySelect 2016, 1, 4987–4993. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Song, L.; Zhang, S.; Wu, X.; Wei, Q. A metal-free and graphitic carbon nitride sonocatalyst with high sonocatalytic activity for degradation methylene blue. Chem. Eng. J. 2012, 184, 256–260. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Abazari, R.; Mahjoub, A.R.; Sanati, S.; Rezvani, Z.; Hou, Z.; Dai, H. Ni–Ti layered double hydroxide@ graphitic carbon nitride nanosheet: A novel nanocomposite with high and ultrafast sonophotocatalytic performance for degradation of antibiotics. Inorg. Chem. 2019, 58, 1834–1849. [Google Scholar] [CrossRef]
- Kong, J.; Lai, X.; Rui, Z.; Ji, H.; Ji, S. Multichannel charge separation promoted ZnO/P25 heterojunctions for the photocatalytic oxidation of toluene. Chin. J. Catal. 2016, 37, 869–877. [Google Scholar] [CrossRef]
- Nirumand, L.; Farhadi, S.; Zabardasti, A.; Khataee, A. Copper ferrite nanoparticles supported on MIL-101/reduced graphene oxide as an efficient and recyclable sonocatalyst. J. Taiwan Inst. Chem. Eng. 2018, 93, 674–685. [Google Scholar] [CrossRef]
- Ghobadifard, M.; Farhadi, S.; Mohebbi, S. Sonocatalytic performance of magnetic flower-like CoFe2O4 nanoparticles prepared from a heterometallic oxo-centered trinuclear complex under microwave irradiation. Polyhedron 2018, 155, 66–76. [Google Scholar] [CrossRef]
- López, Y.O.; Vázquez, H.M.; Gutiérrez, J.S.; Velderrain, V.G.; Ortiz, A.L.; Martínez, V.C. Synthesis method effect of CoFe2O4 on its photocatalytic properties for H2 production from water and visible light. J. Nanomater. 2015, 16, 76. [Google Scholar]
- Safari, J.; Zarnegar, Z. Brønsted acidic ionic liquid based magnetic nanoparticles: A new promoter for the Biginelli synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones. New J. Chem. 2014, 38, 358–365. [Google Scholar] [CrossRef]
- Nivetha, R.; Chella, S.; Kollu, P.; Jeong, S.K.; Bhatnagar, A.; Andrews, N.G. Cobalt and nickel ferrites based graphene nanocomposites for electrochemical hydrogen evolution. J. Magn. Magn. Mater. 2018, 448, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.; Zeng, T.; He, Y.; Zhang, D.; Pu, X. A novel magnetically separable CoFe2O4/Cd0.9Zn0.1S photocatalyst with remarkably enhanced H2 evolution activity under visible light irradiation. Chem. Eng. J. 2019, 359, 485–495. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, D.; Diao, Z.; Wang, M.; Shen, S. Ferrites boosting photocatalytic hydrogen evolution over graphitic carbon nitride: A case study of (Co, Ni)Fe2O4 modification. Sci. Bull. 2016, 61, 292–301. [Google Scholar] [CrossRef] [Green Version]
- Zhong, M.; Fei, P.; Fu, X.; Lei, Z.; Su, B. Synthesis of PS–CoFe2O4 composite nanomaterial with improved magnetic properties by a one-step solvothermal method. Ind. Eng. Chem. Res. 2013, 52, 8230–8235. [Google Scholar] [CrossRef]
- Thomas, B.; Alexander, L. Enhanced synergetic effect of Cr (VI) ion removal and anionic dye degradation with superparamagnetic cobalt ferrite meso–macroporous nanospheres. Appl. Nanosci. 2018, 8, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Xu, G.; Zhang, M.; Xiong, K.; Meng, P. Synthesis of CoFe2O4/RGO nanocomposites by click chemistry and electromagnetic wave absorption properties. J. Mater. Sci. Mater. Electron. 2016, 27, 9278–9285. [Google Scholar] [CrossRef]
- Qin, H.; Lv, W.; Bai, J.; Zhou, Y.; Wen, Y.; He, Q.; Tang, J.; Wang, L.; Zhou, Q. Sulfur-doped porous graphitic carbon nitride heterojunction hybrids for enhanced photocatalytic H2 evolution. J. Mater. Sci. 2019, 54, 4811–4820. [Google Scholar] [CrossRef]
- Joseph, S.; Abraham, S.; Priyanka, R.N.; Abraham, T.; Suresh, A.; Mathew, B. In situ S-doped ultrathin gC3N4 nanosheets coupled with mixed-dimensional (3D/1D) nanostructures of silver vanadates for enhanced photocatalytic degradation of organic pollutants. New J. Chem. 2019, 43, 10618–10630. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Xiao, X.; Guo, L.; Li, Y. Enhanced visible light photocatalytic hydrogen evolution of sulfur-doped polymeric g-C3N4 photocatalysts. Mater. Res. Bull. 2013, 48, 3919–3925. [Google Scholar] [CrossRef]
- Kargar, A.; Yavuz, S.; Kim, T.K.; Liu, C.-H.; Kuru, C.; Rustomji, C.S.; Jin, S.; Bandaru, P.R. Solution-processed CoFe2O4 nanoparticles on 3D carbon fiber papers for durable oxygen evolution reaction. ACS Appl. Mater. Interfaces 2015, 7, 17851–17856. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Lee, Z.; Chu, K.-W.; Wei, Y.-H. CoFe2O4@ZnS core–shell spheres as magnetically recyclable photocatalysts for hydrogen production. J. Taiwan Inst. Chem. Eng. 2016, 66, 386–393. [Google Scholar] [CrossRef]
- Ke, L.; Li, P.; Wu, X.; Jiang, S.; Luo, M.; Liu, Y.; Le, Z.; Sun, C.; Song, S. Graphene-like sulfur-doped g-C3N4 for photocatalytic reduction elimination of UO22+ under visible Light. Appl. Catal. B Environ. 2017, 205, 319–326. [Google Scholar] [CrossRef]
- Shi, C.; Chen, M.; Han, X.; Bi, Y.; Huang, L.; Zhou, K.; Zheng, Z. Thiacalix[4]arene-supported tetradecanuclear cobalt nanocage cluster as precursor to synthesize CoO/Co9S8@CN composite for supercapacitor application. Inorg. Chem. Front. 2018, 5, 1329–1335. [Google Scholar] [CrossRef]
- Sadiq, M.M.J.; Shenoy, U.S.; Bhat, D.K. Synthesis of BaWO4/NRGO–gC3N4 nanocomposites with excellent multifunctional catalytic performance via microwave approach. Front. Mater. Sci. 2018, 12, 247–263. [Google Scholar] [CrossRef]
- Cao, S.; Fan, B.; Feng, Y.; Chen, H.; Jiang, F.; Wang, X. Sulfur-doped g-C3N4 nanosheets with carbon vacancies: General synthesis and improved activity for simulated solar-light photocatalytic nitrogen fixation. Chem. Eng. J. 2018, 353, 147–156. [Google Scholar] [CrossRef]
- Chang, Q.; Yang, S.; Li, L.; Xue, C.; Li, Y.; Wang, Y.; Hu, S.; Yang, J.; Zhang, F. Loading sulfur and nitrogen co-doped carbon dots onto gC3N4 nanosheets for an efficient photocatalytic reduction of 4-nitrophenol. Dalton Trans. 2018, 47, 6435–6443. [Google Scholar] [CrossRef]
- Xiong, P.; Chen, Q.; He, M.; Sun, X.; Wang, X. Cobalt ferrite–polyaniline heteroarchitecture: A magnetically recyclable photocatalyst with highly enhanced performances. J. Mater. Chem. 2012, 22, 17485–17493. [Google Scholar] [CrossRef]
- Sun, X.; Gao, L.; Guo, C.; Zhang, Y.; Kuang, X.; Yan, T.; Ji, L.; Wei, Q. Sulfur incorporated CoFe2O4/multiwalled carbon nanotubes toward enhanced oxygen evolution reaction. Electrochim. Acta 2017, 247, 843–850. [Google Scholar] [CrossRef]
- Navarro-Pardo, F.; Tong, X.; Selopal, G.S.; Cloutier, S.G.; Sun, S.; Tavares, A.C.; Zhao, H.; Wang, Z.M.; Rosei, F. Graphene oxide/cobalt-based nanohybrid electrodes for robust hydrogen generation. Appl. Catal. B Environ. 2019, 245, 167–176. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, A.A.; Moreno-Trejo, M.B.; Meléndez-Zaragoza, M.J.; Collins-Martínez, V.; López-Ortiz, A.; Martínez-Guerra, E.; Sánchez-Domínguez, M. Spinel-type ferrite nanoparticles: Synthesis by the oil-in-water microemulsion reaction method and photocatalytic water-splitting evaluation. Int. J. Hydrog. Energy 2019, 44, 12421–12429. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Zou, R.; Kang, W.; San Chui, Y.; Yuen, M.F.; Lee, C.-S.; Zhang, W. Layer-stacked cobalt ferrite (CoFe2O4) mesoporous platelets for high-performance lithium ion battery anodes. J. Mater. Chem. A 2015, 3, 6990–6997. [Google Scholar] [CrossRef]
- de Lima Alves, T.M.; Amorim, B.F.; Torres, M.A.M.; Bezerra, C.G.; de Medeiros, S.N.; Gastelois, P.L.; Outon, L.E.F.; de Almeida Macedo, W.A. Wasp-waisted behavior in magnetic hysteresis curves of CoFe2O4 nanopowder at a low temperature: Experimental evidence and theoretical approach. RSC Adv. 2017, 7, 22187–22196. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Lee, L.-K. Catalyst and Process for Direct Catalystic Production of Hydrogen Peroxide, (H2O2). U.S. Patent 6,168,775, 2 January 2001. [Google Scholar]
- Lee, G.; Chu, K.H.; Al-Hamadani, Y.A.; Park, C.M.; Jang, M.; Heo, J.; Her, N.; Kim, D.-H.; Yoon, Y. Fabrication of graphene-oxide/β-Bi2O3/TiO2/Bi2Ti2O7 heterojuncted nanocomposite and its sonocatalytic degradation for selected pharmaceuticals. Chemosphere 2018, 212, 723–733. [Google Scholar] [CrossRef]
- Siadatnasab, F.; Farhadi, S.; Khataee, A. Sonocatalytic performance of magnetically separable CuS/CoFe2O4 nanohybrid for efficient degradation of organic dyes. Ultrason. Sonochem. 2018, 44, 359–367. [Google Scholar] [CrossRef]
- Borgohain, C.; Senapati, K.K.; Sarma, K.; Phukan, P. A facile synthesis of nanocrystalline CoFe2O4 embedded one-dimensional ZnO hetero-structure and its use in photocatalysis. J. Mol. Catal. A Chem. 2012, 363, 495–500. [Google Scholar] [CrossRef]
- Huang, F.; Chen, L.; Wang, H.; Yan, Z. Analysis of the degradation mechanism of methylene blue by atmospheric pressure dielectric barrier discharge plasma. Chem. Eng. J. 2010, 162, 250–256. [Google Scholar] [CrossRef]
- Sajjadi, S.; Khataee, A.; Kamali, M. Sonocatalytic degradation of methylene blue by a novel graphene quantum dots anchored CdSe nanocatalyst. Ultrason. Sonochem. 2017, 39, 676–685. [Google Scholar] [CrossRef]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, S.; Pan, G.-T.; Chong, S.; Yang, T.C.-K. Ultrasonically Induced Sulfur-Doped Carbon Nitride/Cobalt Ferrite Nanocomposite for Efficient Sonocatalytic Removal of Organic Dyes. Processes 2020, 8, 104. https://doi.org/10.3390/pr8010104
Kamal S, Pan G-T, Chong S, Yang TC-K. Ultrasonically Induced Sulfur-Doped Carbon Nitride/Cobalt Ferrite Nanocomposite for Efficient Sonocatalytic Removal of Organic Dyes. Processes. 2020; 8(1):104. https://doi.org/10.3390/pr8010104
Chicago/Turabian StyleKamal, Surabhi, Guan-Ting Pan, Siewhui Chong, and Thomas Chung-Kuang Yang. 2020. "Ultrasonically Induced Sulfur-Doped Carbon Nitride/Cobalt Ferrite Nanocomposite for Efficient Sonocatalytic Removal of Organic Dyes" Processes 8, no. 1: 104. https://doi.org/10.3390/pr8010104
APA StyleKamal, S., Pan, G. -T., Chong, S., & Yang, T. C. -K. (2020). Ultrasonically Induced Sulfur-Doped Carbon Nitride/Cobalt Ferrite Nanocomposite for Efficient Sonocatalytic Removal of Organic Dyes. Processes, 8(1), 104. https://doi.org/10.3390/pr8010104