Analyses for Synthesis Gas from Municipal Solid Waste Gasification under Medium Temperatures
Abstract
:1. Introduction
2. Experiment
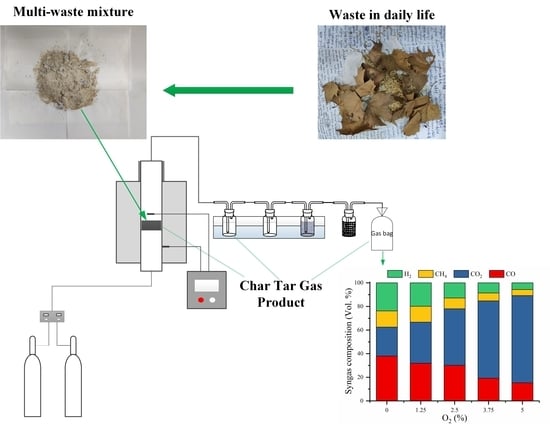
2.1. Material
2.2. Experiment Procedure
2.3. Analysis Method
3. Results and Discussion
3.1. Evolution of Syngas Components
3.2. Effects of Temperature and Oxygen Concentration
3.3. Product Yield and Energy Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- China, National Bureau of Statistics. China Statistical Yearbook 2018, 1st ed.; China Statistics Press: Beijing, China, 2018.
- El-Fadel, M.; Findikakis, A.N.; Leckie, J.O. Environmental Impacts of Solid Waste Landfilling. J. Environ. Manag. 1997, 50, 1–25. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Qiao, Y.; Tian, Y. The steam gasification reactivity and kinetics of municipal solid waste chars derived from rapid pyrolysis. Waste Manag. 2018, 80, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Hoang, A.T.; Bui, X.L.; Pham, X.D. A novel investigation of oil and heavy metal adsorption capacity from as-fabricated adsorbent based on agricultural by-product and porous polymer. Energy Sources Part A Recover. Util. Environ. Effects 2018, 40, 929–939. [Google Scholar] [CrossRef]
- McKay, G. Dioxin characterisation, formation and minimisation during municipal solid waste (MSW) incineration: Review. Chem. Eng. J. 2002, 86, 343–368. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Meng, Y.; Yan, F.; Aihemaiti, A. A review of recent developments in hydrogen production via biogas dry reforming. Energy Convers. Manag. 2018, 171, 133–155. [Google Scholar] [CrossRef]
- Arena, U. Process and technological aspects of municipal solid waste gasification. A review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef]
- Couto, N.D.; Silva, V.B.; Rouboa, A. Assessment on steam gasification of municipal solid waste against biomass substrates. Energy Convers. Manag. 2016, 124, 92–103. [Google Scholar] [CrossRef]
- Couto, N.D.; Silva, V.B.; Rouboa, A. Thermodynamic Evaluation of Portuguese municipal solid waste gasification. J. Clean. Prod. 2016, 139, 622–635. [Google Scholar] [CrossRef]
- Tanigaki, N.; Manako, K.; Osada, M. Co-gasification of municipal solid waste and material recovery in a large-scale gasification and melting system. Waste Manag. 2012, 32, 667–675. [Google Scholar] [CrossRef]
- Bhoi, P.R.; Huhnke, R.L.; Kumar, A.; Indrawan, N.; Thapa, S. Co-gasification of municipal solid waste and biomass in a commercial scale downdraft gasifier. Energy 2018, 163, 513–518. [Google Scholar] [CrossRef]
- Tańczuk, M.; Junga, R.; Werle, S.; Chabiński, M.; Ziółkowski, Ł. Experimental analysis of the fixed bed gasification process of the mixtures of the chicken manure with biomass. Renew. Energy 2019, 136, 1055–1063. [Google Scholar] [CrossRef]
- Materazzi, M.; Taylor, R. Plasma-Assisted Gasification for Waste-to-Fuels Applications. Ind. Eng. Chem. Res. 2019, 58, 15902–15913. [Google Scholar] [CrossRef]
- Fernández-González, J.M.; Grindlay, A.L.; Serrano-Bernardo, F.; Rodríguez-Rojas, M.I.; Zamorano, M. Economic and environmental review of Waste-to-Energy systems for municipal solid waste management in medium and small municipalities. Waste Manag. 2017, 67, 360–374. [Google Scholar] [CrossRef]
- Zhou, H.; Long, Y.; Meng, A.; Li, Q.; Zhang, Y. Classification of municipal solid waste components for thermal conversion in waste-to-energy research. Fuel 2015, 145, 151–157. [Google Scholar] [CrossRef]
- Zhou, H.; Long, Y.; Meng, A.; Li, Q.; Zhang, Y. Interactions of three municipal solid waste components during co-pyrolysis. J. Anal. Appl. Pyrolysis 2015, 111, 265–271. [Google Scholar] [CrossRef]
- Zhou, H.; Long, Y.; Meng, A.; Li, Q.; Zhang, Y. Thermogravimetric characteristics of typical municipal solid waste fractions during co-pyrolysis. Waste Manag. 2015, 38, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Meng, A.; Long, Y.; Li, Q.; Zhang, Y. Interactions of municipal solid waste components during pyrolysis: A TG-FTIR study. J. Anal. Appl. Pyrolysis 2014, 108, 19–25. [Google Scholar] [CrossRef]
- Niu, M.; Huang, Y.; Jin, B.; Wang, X. Oxygen Gasification of Municipal Solid Waste in a Fixed-bed Gasifier. Chin. J. Chem. Eng. 2014, 22, 1021–1026. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, C.; Chang, T.; Weng, W. Waste-gasification efficiency of a two-stage fluidized-bed gasification system. Waste Manag. 2016, 48, 250–256. [Google Scholar] [CrossRef]
- Dong, J.; Chi, Y.; Tang, Y.; Ni, M.; Nzihou, A.; Weiss-Hortala, E.; Huang, Q. Effect of Operating Parameters and Moisture Content on Municipal Solid Waste Pyrolysis and Gasification. Energy Fuel 2015, 30, 3994–4001. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Xiao, B.; Liu, S.; Hu, Z.; Guo, X.; Luo, S.; Yang, F. Syngas production from pyrolysis of municipal solid waste (MSW) with dolomite as downstream catalysts. J. Anal. Appl. Pyrolysis 2010, 87, 181–187. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, W.; Zhang, Y.; Zhou, X. Clean synthesis gas production from municipal solid waste via catalytic gasification and reforming technology. Catal. Today 2018, 318, 39–45. [Google Scholar] [CrossRef]
- Zheng, X.; Ying, Z.; Wang, B.; Chen, C. Hydrogen and syngas production from municipal solid waste (MSW) gasification via reusing CO2. Appl. Therm. Eng. 2018, 144, 242–247. [Google Scholar] [CrossRef]
- Cao, Y.; Fu, L.; Mofrad, A. Combined-gasification of biomass and municipal solid waste in a fluidized bed gasifier. J. Energy Inst. 2019, 92, 1683–1688. [Google Scholar] [CrossRef]
- Lopes, E.J.; Queiroz, N.; Yamamoto, C.I.; Da Costa Neto, P.R. Evaluating the emissions from the gasification processing of municipal solid waste followed by combustion. Waste Manag. 2018, 73, 504–510. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B. The fate of heavy metals during combustion and gasification of contaminated biomass—A brief review. J. Hazard. Mater. 2013, 256–257, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Pei, H.; Wang, X.; Dai, X.; Jin, B.; Huang, Y. A novel two-stage biomass gasification concept: Design and operation of a 1.5 MWth demonstration plant. Bioresour. Technol. 2018, 267, 102–109. [Google Scholar] [CrossRef]
- Niu, M.; Huang, Y.; Jin, B.; Liang, S.; Dong, Q.; Gu, H.; Sun, R. A novel two-stage enriched air biomass gasification for producing low-tar high heating value fuel gas: Pilot verification and performance analysis. Energy 2019, 173, 511–522. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, G.; Huang, R.; Yin, W.; Qing, X.; Ren, M.; Zhang, S. Removal of dioxin in flue gas from a large-scale MSWI by domestic activated carbon injection. Chin. J. Environ. Eng. 2015, 9, 5531–5536. [Google Scholar]
- Wang, H.; Yuan, B.; Hao, R.; Zhao, Y.; Wang, X. A critical review on the method of simultaneous removal of multi-air-pollutant in flue gas. Chem. Eng. J. 2019, 378, 122155. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Yang, Z.; Zhang, Z.; Cai, Z. A field study of polychlorinated dibenzo-p-dioxins and dibenzofurans formation mechanism in a hazardous waste incinerator: Emission reduction strategies. J. Clean. Prod. 2019, 232, 1018–1027. [Google Scholar] [CrossRef]
- Van de Velden, M.; Baeyens, J.; Brems, A.; Janssens, B.; Dewil, R. Fundamentals, kinetics and endothermicity of the biomass pyrolysis reaction. Renew. Energy 2010, 35, 232–242. [Google Scholar] [CrossRef]
- Gómez-Barea, A.; Leckner, B. Modeling of biomass gasification in fluidized bed. Prog. Energy Combust. Sic. 2010, 36, 444–509. [Google Scholar] [CrossRef]
- Valderrama Rios, M.L.; González, A.M.; Lora, E.E.S.; Almazán Del Olmo, O.A. Reduction of tar generated during biomass gasification: A review. Biomass Bioenergy 2018, 108, 345–370. [Google Scholar] [CrossRef]
- Qiao, Y.; Xu, F.; Xu, S.; Yang, D.; Wang, B.; Ming, X.; Hao, J.; Tian, Y. Pyrolysis Characteristics and Kinetics of Typical Municipal Solid Waste Components and Their Mixture: Analytical TG-FTIR Study. Energy Fuel 2018, 32, 10801–10812. [Google Scholar] [CrossRef]
- Wu, L.; Jiang, X.; Lv, G.; Li, X.; Yan, J. Interactive effect of the sorted components of solid recovered fuel manufactured from municipal solid waste by thermogravimetric and kinetic analysis. Waste Manag. 2020, 102, 270–280. [Google Scholar] [CrossRef]
- Butterman, H.C.; Castaldi, M.J.; Gelix, F.; Borrut, D.; Nicol, F.; Lefebvre, B. Biomass and RDF Gasification Using Ballistic Heating TGA Analysis. Waste Biomass Valoriz. 2014, 5, 607–623. [Google Scholar] [CrossRef]
- Ma, W.; Rajput, G.; Pan, M.; Lin, F.; Zhong, L.; Chen, G. Pyrolysis of typical MSW components by Py-GC/MS and TG-FTIR. Fuel 2019, 251, 693–708. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A.; Mohammadi, M.; Mohamed, A.R. Conversion of the greenhouse gas CO2 to the fuel gas CO via the Boudouard reaction: A review. Renew. Sustain. Energy Rev. 2015, 41, 615–632. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, A.; Long, Y.; Li, Q.; Zhang, Y. A review of dioxin-related substances during municipal solid waste incineration. Waste Manag. 2015, 36, 106–118. [Google Scholar] [CrossRef]
- Shen, H.; Wang, Y.; Chyang, C.; Wang, W.; Lin, G. Effects of Sulfur and Calcium Compounds on Dioxin Reduction in a Fluidized Bed Combustor. Aerosol Air Qual. Res. 2019, 19, 1079–1094. [Google Scholar] [CrossRef]
- Násner, A.M.L.; Lora, E.E.S.; Palacio, J.C.E.; Rocha, M.H.; Restrepo, J.C.; Venturini, O.J.; Ratner, A. Refuse Derived Fuel (RDF) production and gasification in a pilot plant integrated with an Otto cycle ICE through Aspen plus™ modelling: Thermodynamic and economic viability. Waste Manag. 2017, 69, 187–201. [Google Scholar] [CrossRef]
- Hu, B.; Huang, Q.; Buekens, A.; Chi, Y.; Yan, J. Co-gasification of municipal solid waste with high alkali coal char in a three-stage gasifier. Energy Convers. Manag. 2017, 153, 473–481. [Google Scholar] [CrossRef]
- Yang, L.; Ge, X.; Wan, C.; Yu, F.; Li, Y. Progress and perspectives in converting biogas to transportation fuels. Renew. Sustain. Energy Rev. 2014, 40, 1133–1152. [Google Scholar] [CrossRef]
- Niu, M.; Huang, Y.; Jin, B.; Wang, X. Simulation of Syngas Production from Municipal Solid Waste Gasification in a Bubbling Fluidized Bed Using Aspen Plus. Ind. Eng. Chem. Res. 2013, 52, 14768–14775. [Google Scholar] [CrossRef]
- Shayan, E.; Zare, V.; Mirzaee, I. Hydrogen production from biomass gasification; a theoretical comparison of using different gasification agents. Energy Convers. Manag. 2018, 159, 30–41. [Google Scholar] [CrossRef]
- Wang, B.; Albarracín-Suazo, S.; Pagán-Torres, Y.; Nikolla, E. Advances in methane conversion processes. Catal. Today 2017, 285, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Vounatsos, P.; Atsonios, K.; Itskos, G.; Agraniotis, M.; Grammelis, P.; Kakaras, E. Classification of Refuse Derived Fuel (RDF) and Model Development of a Novel Thermal Utilization Concept Through Air-Gasification. Waste Biomass Valorization 2016, 7, 1297–1308. [Google Scholar] [CrossRef]
- Yu, M.; Li, Y.; Fan, Y.; Li, R.; Chi, Y. Gasification Characteristics of Combustible Solid Waste with Additives and Kinetics Study. Waste Biomass Valoriz. 2018, 9, 2571–2578. [Google Scholar] [CrossRef]
- Arena, U.; Di Gregorio, F.; De Troia, G.; Saponaro, A. A techno-economic evaluation of a small-scale fluidized bed gasifier for solid recovered fuel. Fuel Process. Technol. 2015, 131, 69–77. [Google Scholar] [CrossRef]






| Temperature (°C) | Oxygen Concentration (%) | Product Distribution | ||
|---|---|---|---|---|
| Syngas (L) | Char (g) | Tar (g) | ||
| 550 | 0 | 0.588 | 0.679 | 0.532 |
| 1.25 | 0.948 | 0.615 | 0.496 | |
| 2.5 | 1.224 | 0.489 | 0.410 | |
| 3.75 | 1.615 | 0.347 | 0.322 | |
| 5 | 1.849 | 0.24 | 0.256 | |
| 600 | 0 | 0.77 | 0.639 | 0.431 |
| 1.25 | 1.109 | 0.56 | 0.391 | |
| 2.5 | 1.401 | 0.448 | 0.314 | |
| 3.75 | 1.755 | 0.322 | 0.228 | |
| 5 | 2.007 | 0.216 | 0.167 | |
| 650 | 0 | 0.898 | 0.59 | 0.365 |
| 1.25 | 1.366 | 0.544 | 0.329 | |
| 2.5 | 1.609 | 0.437 | 0.266 | |
| 3.75 | 1.881 | 0.316 | 0.195 | |
| 5 | 2.111 | 0.19 | 0.142 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Q.; Wu, W.; Jin, B.; Zhou, Z. Analyses for Synthesis Gas from Municipal Solid Waste Gasification under Medium Temperatures. Processes 2020, 8, 84. https://doi.org/10.3390/pr8010084
Gu Q, Wu W, Jin B, Zhou Z. Analyses for Synthesis Gas from Municipal Solid Waste Gasification under Medium Temperatures. Processes. 2020; 8(1):84. https://doi.org/10.3390/pr8010084
Chicago/Turabian StyleGu, Qinyang, Wei Wu, Baosheng Jin, and Zheng Zhou. 2020. "Analyses for Synthesis Gas from Municipal Solid Waste Gasification under Medium Temperatures" Processes 8, no. 1: 84. https://doi.org/10.3390/pr8010084
APA StyleGu, Q., Wu, W., Jin, B., & Zhou, Z. (2020). Analyses for Synthesis Gas from Municipal Solid Waste Gasification under Medium Temperatures. Processes, 8(1), 84. https://doi.org/10.3390/pr8010084