Biosorption of Co2+ Ions from Aqueous Solution by K2HPO4-Pretreated Duckweed Lemna gibba
Abstract
:1. Introduction
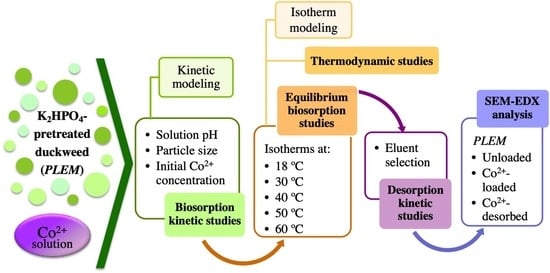
2. Materials and Methods
2.1. Reagents
2.2. Preparation of the Biosorbent
2.3. The Influence of Different Physicochemical Parameters on the Biosorption of Co2+ by PLEM
2.4. Kinetic Modeling of the Biosorption of Co2+ by PLEM
2.5. Biosorption Isotherm Studies at Different Temperatures
2.6. Determination of the Thermodynamic Parameters
2.7. Desorption of Co2+ from the Biosorbent
2.8. Biosorption-Desorption Cycles
2.9. Scanning Electron Microscope Coupled to Energy-Dispersive X-ray Spectroscopy (SEM-EDX)
2.10. Analytical Methods
2.11. Statistical Analysis
3. Results and Discussion
3.1. The Effect of pH
3.2. The Effect of Particle Size
3.3. The Effect of the Initial Co2+ Concentration
3.4. Biosorption Isotherm Studies at Various Temperatures
3.5. Thermodynamic Parameters
3.6. Desorption
3.7. Biosorption-Desorption Cycles
3.8. Scanning Electron Microscopy Coupled with Energy-Dispersive X-ray Spectroscopy (SEM-EDX)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vilvanathan, S.; Shanthakumar, S. Modeling of fixed-bed column studies for removal of cobalt ions from aqueous solution using Chrysanthemum indicum. Res. Chem. Intermed. 2017, 43, 229–243. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Cobalt and Inorganic Cobalt Compounds. In Concise International Chemical Assessment Document 69; Kim, J.H., Gibb, H.J., Howe, P.D., Eds.; WHO: Geneva, Switzerland, 2006; pp. 1–84. Available online: https://apps.who.int/iris/handle/10665/43426 (accessed on 3 August 2020).
- He, E.-K.; Baas, J.; Van Gestel, C.A. Interaction between nickel and cobalt toxicity in Enchytraeus crypticusis due to competitive uptake. Environ. Toxicol. Chem. 2015, 34, 328–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oguz, E.; Ersoy, M. Biosorption of cobalt(II) with sunflower biomass from aqueous solutions in a fixed bed column and neural networks modelling. Ecotoxicol. Environ. Saf. 2014, 99, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.; Deepa, J.; Christa, J. Nanocellulose/nanobentonite composite anchored with multi-carboxyl functional groups as an adsorbent for the effective removal of Cobalt(II) from nuclear industry wastewater samples. J. Colloid Interface Sci. 2016, 467, 307–320. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Hifney, A.F.; Adam, M.S.; Al-Badaani, A.A. Biosorption of cobalt and its effect on growth and metabolites of Synechocystis pevalekii and Scenedesmus bernardii: Isothermal analysis. Environ. Technol. Innov. 2020, 19, 100953. [Google Scholar] [CrossRef]
- Pipíška, M.; Trajteľová, Z.; Horník, M. Compartmentalization of Co and Mn in live cells of Escherichia coli: Investigation using 60Co and 54Mn as radioindicators. J. Radioanal. Nucl. Chem. 2017, 314, 1197–1205. [Google Scholar] [CrossRef]
- Pipíška, M.; Richveisová, B.M.; Frišták, V.; Horník, M.; Remenárová, L.; Stiller, R.; Soja, G. Sorption separation of cobalt and cadmium by straw-derived biochar: A radiometric study. J. Radioanal. Nucl. Chem. 2017, 311, 85–97. [Google Scholar] [CrossRef]
- Pipíška, M.; Zarodňanská, S.; Horník, M.; Ďuriška, L.; Holub, M.; Safarik, I. Magnetically Functionalized Moss Biomass as Biosorbent for Efficient Co2+ Ions and Thioflavin T Removal. Materials 2020, 13, 3619. [Google Scholar] [CrossRef] [PubMed]
- Robalds, A.; Naja, G.M.; Klavins, M. Highlighting inconsistencies regarding metal biosorption. J. Hazard. Mater. 2016, 304, 553–556. [Google Scholar] [CrossRef]
- Saman, N.; Tan, J.-W.; Mohtar, S.S.; Kong, H.; Lye, J.W.P.; Mat, H.; Hassan, H.; Mat, H. Selective biosorption of aurum(III) from aqueous solution using oil palm trunk (OPT) biosorbents: Equilibrium, kinetic and mechanism analyses. Biochem. Eng. J. 2018, 136, 78–87. [Google Scholar] [CrossRef]
- Moradi, P.; Hayati, S.; Ghahrizadeh, T. Modeling and optimization of lead and cobalt biosorption from water with Rafsanjan pistachio shell, using experiment based models of ANN and GP, and the grey wolf optimizer. Chemom. Intell. Lab. Syst. 2020, 202, 104041. [Google Scholar] [CrossRef]
- Muller da Cunha, J.; Klein, L.; Moro Bassaco, M.; Hiromitsu Tanabe, E.; Bertuol, D.A.; Dotto, G.L. Cobalt recovery from leached solutions of lithium-ion batteries using waste materials as adsorbents. Can. J. Chem. Eng. 2015, 93, 2198–2204. [Google Scholar] [CrossRef]
- Greenberg, B.M.; Huang, X.-D.; Dixon, D.G. Applications of the aquatic higher plant Lemna gibba for ecotoxicological assessment. J. Aquat. Ecosyst. Health 1992, 1, 147–155. [Google Scholar] [CrossRef]
- Arroyave, M.D. La lenteja de agua (Lemna minor L.): Una planta acuática promisoria. Rev. EIA 2004, 1, 33–38. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S1794-12372004000100004&lng=en&tlng=es (accessed on 9 November 2020).
- Canales-Gutiérrez, Á. Evaluación de la biomasa y manejo de Lemna gibba (Lenteja de agua) en la bahía interior del lago titicaca, puno. Ecol. Apl. 2010, 9, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Ledezma, J.L. Biosorción de Co(II) a Partir de Soluciones Acuosas por Lemna sp. Master’s Thesis, Instituto Politécnico Nacional, Mexico City, Mexico, 2017. [Google Scholar]
- Reyes-Ledezma, J.L.; Uribe-Ramírez, D.; Cristiani-Urbina, E.; Morales-Barrera, L. Biosorptive removal of acid orange 74 dye by HCl-pretreated Lemna sp. PLoS ONE 2020, 15, e0228595. [Google Scholar] [CrossRef]
- Morales-Barrera, L.; Flores-Ortiz, C.M.; Cristiani-Urbina, E. Single and Binary Equilibrium Studies for Ni2+ and Zn2+ Biosorption onto Lemna Gibba from Aqueous Solutions. Processes 2020, 8, 1089. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, S.; Kumar, A. Biosorption of Ni(II) ions from aqueous solution using modified Aloe barbadensis Miller leaf powder. Water Sci. Eng. 2019, 12, 27–36. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, X.; Huang, H. Kinetics and Thermodynamics of Reserpine Adsorption onto Strong Acidic Cationic Exchange Fiber. PLoS ONE 2015, 10, e0138619. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhao, X.; Deng, J.; He, C. Utilization of chitosan–clinoptilolite composite for the removal of radiocobalt from aqueous solution: Kinetics and thermodynamics. J. Radioanal. Nucl. Chem. 2016, 308, 701–709. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, A.E.; Reyes-Ledezma, J.L.; Chávez-Camarillo, G.M.; Cristiani-Urbina, E.; Morales-Barrera, L. Cyclic biosorption and desorption of acid red 27 onto Eichhornia crassipes leaves. Rev. Mex. Ing. Química 2018, 17, 1121–1134. [Google Scholar] [CrossRef]
- Gramlich, A.; Moradi, A.B.; Robinson, B.H.; Kaestner, A.; Schulin, R. Dimethylglyoxime (DMG) staining for semi-quantitative mapping of Ni in plant tissue. Environ. Exp. Bot. 2011, 71, 232–240. [Google Scholar] [CrossRef]
- Yu, H.; Pang, J.; Ai, T.; Liu, L. Biosorption of Cu2+, Co2+ and Ni2+ from aqueous solution by modified corn silk: Equilibrium, kinetics, and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2016, 62, 21–30. [Google Scholar] [CrossRef]
- Tofan, L.; Teodosiu, C.; Paduraru, C.; Wenkert, R. Cobalt (II) removal from aqueous solutions by natural hemp fibers: Batch and fixed-bed column studies. Appl. Surf. Sci. 2013, 285 Pt A, 33–39. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Hong, K.-J.; Kajiuchi, T.; Yang, J.-W. Determination of the efficiency and removal mechanism of cobalt by crab shell particles. J. Chem. Technol. Biotechnol. 2004, 79, 1388–1394. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Y.; Tay, J.-H. Effect of pH on nickel biosorption by aerobic granular sludge. Bioresour. Technol. 2006, 97, 359–363. [Google Scholar] [CrossRef]
- Suazo-Madrid, A.; Morales-Barrera, L.; Aranda-García, E.; Cristiani-Urbina, E. Nickel(II) biosorption by Rhodotorula glutinis. J. Ind. Microbiol. Biotechnol. 2011, 38, 51–64. [Google Scholar] [CrossRef]
- Abbas, M.; Kaddour, S.; Trari, M. Kinetic and equilibrium studies of cobalt adsorption on apricot stone activated carbon. J. Ind. Eng. Chem. 2014, 20, 745–751. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.-S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Volesky, B. Detoxification of metal-bearing effluents: Biosorption for the next century. Hydrometallurgy 2001, 59, 203–216. [Google Scholar] [CrossRef]
- Bulgariu, D.; Bulgariu, L. Potential use of alkaline treated algae waste biomass as sustainable biosorbent for clean recovery of cadmium(II) from aqueous media: Batch and column studies. J. Clean. Prod. 2016, 112, 4525–4533. [Google Scholar] [CrossRef]
- Mishra, A.; Tripathi, B.D.; Rai, A.K. Packed-bed column biosorption of chromium(VI) and nickel(II) onto Fenton modified Hydrilla verticillata dried biomass. Ecotoxicol. Environ. Saf. 2016, 132, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Vilvanathan, S.; Shanthakumar, S. Biosorption of Co(II) ions from aqueous solution using Chrysanthemum indicum: Kinetics, equilibrium and thermodynamics. Process. Saf. Environ. Prot. 2015, 96, 98–110. [Google Scholar] [CrossRef]
- Kılıç, M.; Kırbıyık, Ç.; Çepelioğullar, Ö.; Putun, A.E. Adsorption of heavy metal ions from aqueous solutions by bio-char, a by-product of pyrolysis. Appl. Surf. Sci. 2013, 283, 856–862. [Google Scholar] [CrossRef]
- Thilagavathy, P.; Santhi, T. Kinetics, Isotherms and Equilibrium Study of Co(II) Adsorption from Single and Binary Aqueous Solutions by Acacia nilotica Leaf Carbon. Chin. J. Chem. Eng. 2014, 22, 1193–1198. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Anu, N.; Nandagopal, M.S.G.; Selvaraju, N. Relevance of isotherm models in biosorption of pollutants by agricultural byproducts. J. Environ. Chem. Eng. 2014, 2, 398–414. [Google Scholar] [CrossRef]
- Hymavathi, D.; Prabhakar, G. Optimization, equilibrium, and kinetic studies of adsorptive removal of cobalt(II) from aqueous solutions using Cocos nucifera L. Chem. Eng. Commun. 2017, 204, 1094–1104. [Google Scholar] [CrossRef]
- Imessaoudene, D.; Hanini, S.; Bouzidi, A.; Ararem, A. Kinetic and thermodynamic study of cobalt adsorption by spent coffee. Desalin. Water Treat. 2015, 57, 6116–6123. [Google Scholar] [CrossRef]
- Marešová, J.; Pipíška, M.; Rozložník, M.; Horník, M.; Remenárová, L.; Augustín, J. Cobalt and strontium sorption by moss biosorbent: Modeling of single and binary metal systems. Desalination 2011, 266, 134–141. [Google Scholar] [CrossRef]
- Demirbaş, E. Adsorption of Cobalt(II) Ions from Aqueous Solution onto Activated Carbon Prepared from Hazelnut Shells. Adsorpt. Sci. Technol. 2003, 21, 951–963. [Google Scholar] [CrossRef]
- Lucaci, A.R.; Bulgariu, D.; Ahmad, I.; Bulgariu, L. Equilibrium and Kinetics Studies of Metal Ions Biosorption on Alginate Extracted from Marine Red Algae Biomass (Callithamnion corymbosum sp.). Polymers 2020, 12, 1888. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Jegan, J.; Palanivelu, K.; Velan, M. Biosorption of cobalt(II) and nickel(II) by seaweeds: Batch and column studies. Sep. Purif. Technol. 2005, 44, 53–59. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Minocha, A.; Sillanpää, M. Adsorptive removal of cobalt from aqueous solution by utilizing lemon peel as biosorbent. Biochem. Eng. J. 2010, 48, 181–186. [Google Scholar] [CrossRef]
- Lakshmipathy, R.; Sarada, N. Application of watermelon rind as sorbent for removal of nickel and cobalt from aqueous solution. Int. J. Miner. Process. 2013, 122, 63–65. [Google Scholar] [CrossRef]
- Vilvanathan, S.; Shanthakumar, S. Removal of Ni(II) and Co(II) ions from aqueous solution using teak (Tectona grandis) leaves powder: Adsorption kinetics, equilibrium and thermodynamics study. Desalin. Water Treat. 2016, 57, 3995–4007. [Google Scholar] [CrossRef]
- Dabbagh, R.; Moghaddam, Z.A.; Ghafourian, H. Removal of cobalt(II) ion from water by adsorption using intact and modified Ficus carica leaves as low-cost natural sorbent. Desalin. Water Treat. 2016, 57, 19890–19902. [Google Scholar] [CrossRef]
- Singh, S.A.; Shukla, S.R. Adsorptive removal of cobalt ions on raw and alkali-treated lemon peels. Int. J. Environ. Sci. Technol. 2016, 13, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Ahmadpour, A.; Tahmasbi, M.; Bastami, T.R.; Besharati, J.A. Rapid removal of cobalt ion from aqueous solutions by almond green hull. J. Hazard. Mater. 2009, 166, 925–930. [Google Scholar] [CrossRef]
- Nadeem, R.; Zafar, M.N.; Afzal, A.; Hanif, M.A.; Saeed, R. Potential of NaOH pretreated Mangifera indica waste biomass for the mitigation of Ni(II) and Co(II) from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2014, 45, 967–972. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Removal of Cu(II) by natural and acid-activated clays: An insight of adsorption isotherm, kinetic and thermodynamics. Desalination 2011, 272, 66–75. [Google Scholar] [CrossRef]
- Ye, W.-M.; He, Y.; Chen, Y.-G.; Chen, B.; Cui, Y.J. Adsorption, Desorption and Competitive Adsorption of Heavy Metal Ions from Aqueous Solution onto GMZ01 Bentonite. In Engineering Geology for Society and Territory; Lollino, G., Giordan, D., Thuro, K., Carranza-Torres, C., Wu, F., Marinos, P., Delgado, C., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 6, pp. 533–536. [Google Scholar]
- Zhang, X.; Wang, X. Adsorption and Desorption of Nickel(II) Ions from Aqueous Solution by a Lignocellulose/Montmorillonite Nanocomposite. PLoS ONE 2015, 10, e0117077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronda, A.; Calero, M.; Blázquez, G.; Pérez, A.; Martín-Lara, M.Á. Optimization of the use of a biosorbent to remove heavy metals: Regeneration and reuse of exhausted biosorbent. J. Taiwan Inst. Chem. Eng. 2015, 51, 109–118. [Google Scholar] [CrossRef]






| Kinetic Models | Equation | Parameters |
|---|---|---|
| Pseudo-first-order [18] | k1—pseudo-first-order sorption velocity constant (min−1) qeq1—equilibrium biosorption capacity predicted by the model (mg g−1) | |
| Pseudo-second-order [18] | k2—pseudo-second-order sorption velocity constant (g mg−1 min−1) qeq2—equilibrium biosorption capacity predicted by the model (mg g−1) | |
| Fractional power [18] | kFP—constant of the model (mg g−1) ν—velocity constant (h−1) | |
| Isothermal models | Equation | Parameters |
| Langmuir [19,20] | qmL—maximum biosorption capacity determined by Langmuir (mg g−1) bL—Langmuir constant, linked to affinity for the active sites (L mg−1) Cini—initial concentration (mg L−1) RL—separation factor | |
| Freundlich [19] | kF—Freundlich constant, related to the biosorption capacity (mg g−1 (mg L−1)−1/nF) nF—Freundlich constant, linked to the intensity of sorption | |
| Sips [19] | qmSP—maximum biosorption capacity, determined by Sips (mg g−1) kSP—constant of the model (mg L−1)−nSP nSP—exponent of the model | |
| Redlich-Peterson [19] | kRP—constant of the model (L g−1) aRP—constant of the model (mg L−1) −bRP bRP—exponent of the model |
| Parameter | pH | |||
|---|---|---|---|---|
| 4.0 | 5.0 | 6.0 | 7.0 | |
| qeq (mg g−1) | 12.35 ± 0.09 | 17.85 ± 0.08 | 29.78 ± 0.18 | 40.13 ± 0.18 |
| teq (h) | 0.25 | 0.25 | 0.25 | 0.5 |
| Pseudo-first-order | ||||
| qeq1 (mg g−1) | 12.19 ± 0.15 | 17.51 ± 0.13 | 29.20 ± 0.22 | 38.80 ± 0.26 |
| k1 (h−1) | 18.81 ± 0.98 | 25.03 ± 1.11 | 41.79 ± 2.32 | 34.96 ± 1.59 |
| R2 | 0.9740 | 0.9757 | 0.9699 | 0.9777 |
| ASE | 17.570 | 33.360 | 104.200 | 139.400 |
| Sy.x | 0.6468 | 0.7860 | 1.389 | 1.607 |
| AICc | −33.79 | −22.54 | 41.25 | 57.55 |
| Pseudo-second-order | ||||
| qeq2 (mg g−1) | 13.69 ± 0.22 | 18.61 ± 0.11 | 30.57 ± 0.16 | 40.86 ± 0.09 |
| k2 (g mg−1 h−1) | 1.88 ± 0.17 | 2.28 ± 0.10 | 2.51 ± 0.13 | 1.50 ± 0.03 |
| R2 | 0.975 | 0.990 | 0.989 | 0.998 |
| ASE | 16.81 | 13.75 | 35.23 | 12.31 |
| Sy.x | 0.6327 | 0.5046 | 0.8077 | 0.4775 |
| AICc | −35.73 | −72.18 | −19.50 | −78.37 |
| Fractional power | ||||
| kFP (mg g−1) | 15.03 ± 0.56 | 18.37 ± 0.33 | 30.61 ± 0.39 | 40.85 ± 0.52 |
| v (h−1) | 0.211 ± 0.02 | 0.107 ± 0.01 | 0.073 ± 0.007 | 0.086 ± 0.008 |
| R2 | 0.758 | 0.676 | 0.667 | 0.7293 |
| ASE | 68.31 | 133.90 | 190.00 | 342.90 |
| Sy.x | 1.341 | 1.637 | 1.949 | 2.619 |
| AICc | 55.70 | 80.49 | 73.88 | 104.6 |
| Parameter | Particle Size (mm) | ||||
|---|---|---|---|---|---|
| 0.3–0.5 | 0.5–0.8 | 0.8–1.4 | 1.4–2 | 0.3–0.8 | |
| qeq (mg g−1) | 40.13 ± 0.18 | 39.77 ± 0.25 | 33.38 ± 0.17 | 32.25 ± 0.07 | 40.05 ± 0.16 |
| teq (h) | 0.5 | 0.75 | 1.0 | 1.0 | 0.5 |
| Pseudo-first-order | |||||
| qeq1 (mg g−1) | 38.80 ± 0.26 | 38.01 ± 0.29 | 31.74 ± 0.32 | 30.91 ± 0.31 | 38.76 ± 0.25 |
| k1 (h−1) | 34.96 ± 1.59 | 36.02 ± 1.98 | 13.66 ± 0.62 | 12.41 ± 0.52 | 35.08 ± 1.56 |
| R2 | 0.978 | 0.968 | 0.969 | 0.973 | 0.979 |
| ASE | 139.4 | 191.9 | 154.2 | 130.8 | 134.0 |
| Sy.x | 1.607 | 1.885 | 1.690 | 1.556 | 1.575 |
| AICc | 57.55 | 75.43 | 63.20 | 53.97 | 55.32 |
| Pseudo-second-order | |||||
| qeq2 (mg g−1) | 40.86 ± 0.09 | 40.07 ± 0.10 | 34.45 ± 0.23 | 33.64 ± 0.19 | 40.80 ±0.09 |
| k2 (g mg−1 h−1) | 1.50 ± 0.03 | 1.55 ± 0.04 | 0.61 ± 0.02 | 0.56 ± 0.02 | 1.52 ± 0.09 |
| R2 | 0.998 | 0.998 | 0.991 | 0.994 | 0.998 |
| ASE | 12.31 | 14.66 | 46.04 | 27.98 | 11.73 |
| Sy.x | 0.478 | 0.521 | 0.923 | 0.720 | 0.466 |
| AICc | −78.37 | −68.58 | −4.504 | −32.39 | −81.09 |
| Fractional power | |||||
| kFP (mg g−1) | 40.85 ± 0.52 | 40.18 ± 0.45 | 33.06 ± 0.58 | 32.02 ± 0.59 | 40.78 ± 0.52 |
| v (h−1) | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.17 ± 0.01 | 0.18 ± 0.01 | 0.08 ± 0.01 |
| R2 | 0.729 | 0.781 | 0.819 | 0.814 | 0.723 |
| ASE | 342.9 | 256.4 | 422.2 | 453.9 | 348.1 |
| Sy.x | 2.619 | 2.265 | 2.906 | 3.013 | 2.638 |
| AICc | 104.6 | 89.47 | 115.4 | 119.2 | 105.4 |
| Parameter | Cini (mg L−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 40 | 60 | 80 | 100 | 200 | 300 | |
| qeq (mg g−1) | 5.46 ± 0.16 | 12.18 ± 0.20 | 20.20 ± 0.13 | 29.22 ± 0.19 | 36.44 ± 0.48 | 40.05 ± 0.16 | 44.22 ± 0.31 | 46.17 ± 0.41 |
| teq (h) | 0.05 | 0.08 | 0.16 | 0.2 | 0.5 | 0.5 | 0.75 | 0.75 |
| Pseudo-first-order | ||||||||
| qeq1 (mg g−1) | 5.71 ± 0.03 | 12.44 ± 0.07 | 19.95 ± 0.14 | 28.64 ± 0.23 | 34.53 ± 0.41 | 38.76 ± 0.25 | 41.56 ± 0.46 | 43.81 ± 0.42 |
| k1 (h−1) | 24.01 ± 0.85 | 25.00 ± 0.81 | 32.93 ± 1.58 | 34.58 ± 1.91 | 35.81 ± 2.94 | 35.08 ± 1.56 | 33.50 ± 2.53 | 37.68 ± 2.58 |
| R2 | 0.9853 | 0.9871 | 0.9741 | 0.9666 | 0.9288 | 0.9785 | 0.9371 | 0.9514 |
| ASE | 2.324 | 8.894 | 43.08 | 113.9 | 356.2 | 134.0 | 456.9 | 384.9 |
| Sy.x | 0.2075 | 0.4058 | 0.8932 | 1.452 | 2.568 | 1.575 | 2.909 | 2.670 |
| AICc | −171.7 | −96.58 | −8.221 | 46.20 | 110.1 | 55.32 | 124.0 | 114.4 |
| Pseudo-second-order | ||||||||
| qeq2 (mg g−1) | 6.05 ± 0.09 | 13.15 ± 0.09 | 20.97 ± 0.10 | 30.13 ± 0.15 | 36.52 ± 0.27 | 40.80 ± 0.09 | 44.03 ± 0.26 | 46.19 ± 0.23 |
| k2 (g mg−1 h−1) | 6.85 ± 0.76 | 3.37 ± 0.20 | 2.85 ± 0.13 | 2.06 ± 0.09 | 1.65 ± 0.11 | 1.52 ± 0.03 | 1.27 ± 0.07 | 1.40 ± 0.07 |
| R2 | 0.9456 | 0.9830 | 0.9915 | 0.9915 | 0.9804 | 0.9981 | 0.9882 | 0.9912 |
| ASE | 8.617 | 11.74 | 14.17 | 29.02 | 98.25 | 11.73 | 85.78 | 69.72 |
| Sy.x | 0.3995 | 0.4663 | 0.5122 | 0.7331 | 1.349 | 0.4660 | 1.260 | 1.136 |
| AICc | −98.35 | −81.02 | −70.50 | −30.34 | 37.94 | −81.09 | 30.34 | 18.74 |
| Fractional power | ||||||||
| kFP (mg g−1) | 5.89 ± 0.16 | 12.92 ± 0.26 | 20.84 ± 0.32 | 30.05 ± 0.42 | 36.85 ± 0.36 | 40.78 ± 0.53 | 44.33 ± 0.41 | 46.49 ± 0.46 |
| v (h−1) | 0.099 ± 0.02 | 0.099 ± 0.01 | 0.083 ± 0.009 | 0.083 ± 0.008 | 0.095 ± 0.005 | 0.085 ± 0.007 | 0.098 ± 0.005 | 0.088 ± 0.006 |
| R2 | 0.4581 | 0.5935 | 0.6402 | 0.6845 | 0.8463 | 0.7226 | 0.8675 | 0.8184 |
| ASE | 31.46 | 84.08 | 128.5 | 218.8 | 157.8 | 348.1 | 206.0 | 269.9 |
| Sy.x | 0.7932 | 1.297 | 1.603 | 2.092 | 1.777 | 2.638 | 2.030 | 2.323 |
| AICc | −19.63 | 31.48 | 53.53 | 81.21 | 64.23 | 105.4 | 78.08 | 92.13 |
| Parameter | Temperature (°C) | ||||
|---|---|---|---|---|---|
| 18 | 30 | 40 | 50 | 60 | |
| qm exp (mg g−1) | 46.17 ± 0.41 | 46.33 ± 0.19 | 47.73 ± 0.12 | 48.49 ± 0.21 | 49.35 ± 0.22 |
| Langmuir | |||||
| bL (L mg−1) | 0.047 ± 0.005 | 0.046 ± 0.005 | 0.046 ± 0.004 | 0.045 ± 0.003 | 0.048 ± 0.006 |
| qmL (mg g−1) | 51.12 ± 1.49 | 52.66 ± 1.82 | 52.78 ± 1.48 | 54.97 ± 1.27 | 55.57 ± 1.91 |
| RL | 0.508 − 0.068 | 0.509 − 0.073 | 0.508 − 0.075 | 0.513 − 0.076 | 0.498 − 0.071 |
| R2 | 0.993 | 0.990 | 0.993 | 0.996 | 0.990 |
| ASE | 13.65 | 19.18 | 12.9 | 8.856 | 21.46 |
| Sy.x | 1.508 | 1.788 | 1.466 | 1.215 | 1.891 |
| AICc | 16.28 | 18.99 | 15.82 | 12.81 | 19.89 |
| Freundlich | |||||
| kF (mg g−1 (L g−1)1/nF) | 11.05 ± 2.65 | 10.82 ± 2.66 | 10.6 ± 2.39 | 10.94 ± 2.43 | 11.71 ± 2.80 |
| nF | 3.64 ± 0.70 | 3.504 ± 0.67 | 3.433 ± 0.59 | 3.422 ± 0.58 | 3.53 ± 0.67 |
| R2 | 0.924 | 0.922 | 0.938 | 0.939 | 0.926 |
| ASE | 140.7 | 150.4 | 120.7 | 126 | 161 |
| Sy.x | 4.842 | 5.006 | 4.484 | 4.582 | 5.179 |
| AICc | 34.94 | 35.47 | 33.71 | 34.05 | 36.01 |
| Sips | |||||
| kSP (L g−1) | 0.022 ± 0.005 | 0.018 ± 0.005 | 0.022 ± 0.005 | 0.026 ± 0.004 | 0.023 ± 0.008 |
| qmSP (mg g−1) | 47.55 ± 1.026 | 48.25 ± 1.065 | 48.79 ± 1.122 | 51.57 ± 0.975 | 51.55 ± 1.677 |
| nSP | 1.295 ± 0.086 | 1.367 ± 0.095 | 1.294 ± 0.092 | 1.224 ± 0.066 | 1.304 ± 0.131 |
| R2 | 0.998 | 0.998 | 0.998 | 0.999 | 0.996 |
| ASE | 3.713 | 4.38 | 4.055 | 2.504 | 9.674 |
| Sy.x | 0.8617 | 0.936 | 0.900 | 0.7077 | 1.391 |
| AICc | 15.19 | 16.51 | 15.9 | 12.04 | 22.85 |
| Redlich-Peterson | |||||
| kRP (L g−1) | 1.822 ± 0.096 | 1.789 ± 0.126 | 2.006 ± 0.222 | 1.964 ± 0.099 | 1.996 ± 0.178 |
| aRP (L mg−1)bRP | 0.017 ± 0.003 | 0.014 ± 0.004 | 0.023 ± 0.009 | 0.019 ± 0.004 | 0.015 ± 0.006 |
| bRP | 1.142 ± 0.029 | 1.171 ± 0.044 | 1.095 ± 0.057 | 1.116 ± 0.028 | 1.162 ± 0.054 |
| R2 | 0.999 | 0.998 | 0.996 | 0.999 | 0.997 |
| ASE | 2.111 | 4.197 | 7.774 | 1.824 | 7.069 |
| Sy.x | 0.6498 | 0.9162 | 1.247 | 0.604 | 1.189 |
| AICc | 10.68 | 16.17 | 21.1 | 9.507 | 20.34 |
| Material | Biosorption Capacity (mg g−1) | pH | Reference |
|---|---|---|---|
| Cocos nucifera leaf | 3.69 | 5 | [39] |
| Spent coffee | 5.37 | 6 | [40] |
| Rhytidiadelphus squarrosus | 7.38 | 6 | [41] |
| Natural hemp fibers | 13.58 | 4.5–5 | [26] |
| Activated carbon from hazelnut shells | 13.88 | 6 | [42] |
| Alginate from Callithamnion corymbosum sp. | 18.79 | 4.4 | [43] |
| Sargassum wightii | 20.63 | 4.5 | [44] |
| Carbonized lemon peel | 22.00 | 6 | [45] |
| Watermelon rind | 23.30 | 5 | [46] |
| Prunus dulcis bio-char | 27.86 | 7 | [36] |
| Teak leaves | 29.48 | 5 | [47] |
| MgCl2-pretreated Ficus carica leaves | 33.9 | 6 | [48] |
| Acacia nilotica | 35.45 | 5 | [37] |
| NaOH-treated lemon peels | 35.71 | 6 | [49] |
| Almond green hull | 45.50 | ND | [50] |
| PLEM | 46.47 | 7 | The present study |
| Corn silk | 82.04 | 6 | [25] |
| NaOH-pretreated Mangifera indica leaves | 114 | 5 | [51] |
| Temperature °C | ΔG0 (J mol−1) | ΔH0 (J mol−1) | ΔS0 (J mol−1 K−1) |
|---|---|---|---|
| 18 | −684.8 | 2461.3 | 10.81 ± 1.89 |
| 30 | −804.3 | 2471.6 | |
| 40 | −825.0 | 2558.8 | |
| 50 | −959.4 | 2532.6 | |
| 60 | −1172.1 | 2427.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Ledezma, J.L.; Cristiani-Urbina, E.; Morales-Barrera, L. Biosorption of Co2+ Ions from Aqueous Solution by K2HPO4-Pretreated Duckweed Lemna gibba. Processes 2020, 8, 1532. https://doi.org/10.3390/pr8121532
Reyes-Ledezma JL, Cristiani-Urbina E, Morales-Barrera L. Biosorption of Co2+ Ions from Aqueous Solution by K2HPO4-Pretreated Duckweed Lemna gibba. Processes. 2020; 8(12):1532. https://doi.org/10.3390/pr8121532
Chicago/Turabian StyleReyes-Ledezma, Jessica Lizeth, Eliseo Cristiani-Urbina, and Liliana Morales-Barrera. 2020. "Biosorption of Co2+ Ions from Aqueous Solution by K2HPO4-Pretreated Duckweed Lemna gibba" Processes 8, no. 12: 1532. https://doi.org/10.3390/pr8121532
APA StyleReyes-Ledezma, J. L., Cristiani-Urbina, E., & Morales-Barrera, L. (2020). Biosorption of Co2+ Ions from Aqueous Solution by K2HPO4-Pretreated Duckweed Lemna gibba. Processes, 8(12), 1532. https://doi.org/10.3390/pr8121532