Synthesis of 2-(4-hydroxyphenyl)ethyl 3,4,5-Trihydroxybenzoate and Its Inhibitory Effect on Sucrase and Maltase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of 2-(4-hydroxyphenethyl)enyl 3,4,5-trihydroxybenzoate (HETB)
2.1.1. Synthesis of 2-[4-(benzyloxy)phenyl]ethanol (3)
2.1.2. Synthesis of methyl 3,4,5-tris(benzyloxy)benzoate (5)
2.1.3. Synthesis of 3,4,5-tris(benzyloxy)benzoic acid (6)
2.1.4. Synthesis of 4-phenoxyphenethyl 3,4,5-triphenoxybenzoate (7)
2.1.5. Synthesis of 2-(4-hydroxyphenethyl)enyl 3,4,5-triphenoxybenzoate (HETB)
2.2. Maltase Activity Assay
2.3. Maltase Inhibitory Activity of HETB and the Intermediate Compounds
2.4. Sucrase Activity Assay
2.5. Sucrase Inhibitory Activity of HETB and the Intermediate Compounds
2.6. Dixon Plots and Lineweaver-Burk Plots
2.7. Docking Experiments
3. Results and Discussion
3.1. Synthesis of HETB
3.2. Maltase and Sucrase Inhibitory Activity of Compounds 1–7 and HETB
3.3. Maltase and Sucrase Inhibitory Activity of Acarbose, Voglibose, and HETB
3.4. Kinetics of Maltase Inhibition by Acarbose, Voglibose, and HETB
3.5. Kinetics of Sucrase Inhibition by Acarbose, Voglibose, and HETB
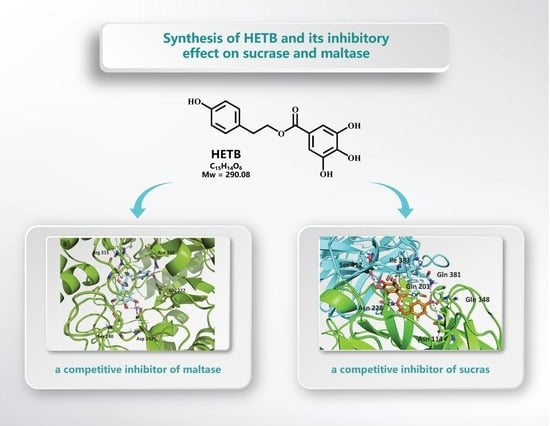
3.6. Molecular Docking of HETB with Maltase and Sucrase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vinodhini, S.; Rajeswari, V.D. Exploring the antidiabetic and anti-obesity properties of Samanea saman through in vitro and in vivo approaches. J. Cell Biochem. 2019, 120, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Chantarasinlapin, P.; Thammarat, H.; Yibchok-Anun, S. A series of cinnamic acid derivatives and their inhibitory activity on intestinal alpha-glucosidase. J. Enzyme Inhib. Med. Chem. 2009, 24, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Rabasa-Lhoret, R.; Chiasson, J.L. Potential of alpha-glucosidase inhibitors in elderly patients with diabetes mellitus and impaired glucose tolerance. Drugs Aging 1998, 13, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Benalla, W.; Bellahcen, S.; Bnouham, M. Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors. Curr. Diabetes Rev. 2010, 6, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Ruengsamran, T.; Kampa, P.; Sompong, W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complement. Altern. Med. 2012, 12, 110. [Google Scholar] [CrossRef] [Green Version]
- Southgate, D.A. Digestion and metabolism of sugars. Am. J. Clin. Nutr. 1995, 62, 203S–210S. [Google Scholar] [CrossRef]
- Ryu, H.J.; Seo, E.S.; Kang, H.K.; Kim, Y.M.; Kim, D. Expression, purification, and characterization of human intestinal maltase secreted from Pichia pastoris. Food Sci. Biotechnol. 2011, 20, 561–565. [Google Scholar] [CrossRef]
- Zheng, Y.G.; Shentu, X.P.; Shen, Y.C. Inhibition of porcine small intestinal sucrase by valienamine. J. Enzyme Inhib. Med. Chem. 2005, 20, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Girish, T.K.; Pratape, V.M.; Prasada Raoa, U.J.S. Nutrient distribution, phenolic acid composition, antioxidant and alpha-glucosidase inhibitory potentials of black gram (Vigna mungo L.) and its milled by-products. Food Res. Int. 2012, 46, 370–377. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Murata, Y.; Inui, H.; Sugiura, M.; Nakano, Y. Triterpene glycosides of Siraitia grosvenori inhibit rat intestinal maltase and suppress the rise in blood glucose level after a single oral administration of maltose in rats. J. Agric. Food Chem. 2005, 53, 2941–2946. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, H.; Nagao, M.; Harada, T.; Nakajima, Y.; Tanimura-Inagaki, K.; Okajima, F.; Tamura, H.; Inazawa, T.; Otonari, T.; Kawakami, M.; et al. Comparison of three α-glucosidase inhibitors for glycemic control and bodyweight reduction in Japanese patients with obese type 2 diabetes. J. Diabetes Investig. 2014, 23, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Taira, M.; Takasu, N.; Komiya, I.; Taira, T.; Tanaka, H. Voglibose administration before the evening meal improves nocturnal hypoglycemia in insulin-dependent diabetic patients with intensive insulin therapy. Metabolism 2000, 49, 440–443. [Google Scholar] [CrossRef]
- Chu, Y.H.; Wu, S.H.; Hsieh, J.F. Isolation and characterization of α-glucosidase inhibitory constituents from Rhodiola crenulata. Food Res. Int. 2014, 57, 8–14. [Google Scholar] [CrossRef]
- Akkarachiyasit, S.; Charoenlertkul, P.; Yibchok-Anun, S.; Adisakwattana, S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int. J. Mol. Sci. 2010, 11, 3387–3396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.T.; Chuang, Y.H.; Hsieh, J.F. Characterization of maltase and sucrase inhibitory constituents from Rhodiola crenulata. Foods 2019, 8, 540. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Miyake, H.; Kusunoki, M.; Osaki, S. Crystal structures of isomaltase from Saccharomyces cerevisiae and in complex with its competitive inhibitor maltose. FEBS J. 2010, 277, 4205–4214. [Google Scholar] [CrossRef]
- Sainz-Polo, M.A.; Ramírez-Escudero, M.; Lafraya, A.; González, B.; Marín-Navarro, J.; Polaina, J.; Sanz-Aparicio, J. Three-dimensional structure of Saccharomyces invertase: Role of a non-catalytic domain in oligomerization and substrate specificity. J. Biol. Chem. 2013, 288, 9755–9766. [Google Scholar] [CrossRef] [Green Version]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Lee, C.W.; Son, E.M.; Kim, H.S.; Xu, P.; Batmunkh, T.; Lee, B.J.; Koo, K.A. Synthetic tyrosyl gallate derivatives as potent melanin formation inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 5462–5464. [Google Scholar] [CrossRef]
- Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Hong, J.T.; Hwang, B.Y.; Jung, J.K.; Lee, J.; Jeong, H.S. Isolation and characterisation of an α-glucosidase inhibitory substance from fructose-tyrosine Maillard reaction products. Food Chem. 2011, 127, 122–126. [Google Scholar] [CrossRef]
- Juretić, D.; Bernik, S.; Cop, L.; Hadzija, M.; Petlevski, R.; Lukac-Bajalo, J. Short-term effect of acarbose on specific intestinal disaccharidase activities and hyperglycaemia in CBA diabetic mice. J. Anim. Physiol. Anim. Nutr. (Berl.) 2003, 87, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Asano, N. Glycosidase inhibitors: Update and perspectives on practical use. Glycobiology 2003, 13, 93R–104R. [Google Scholar] [CrossRef] [PubMed]
- Dabhi, A.S.; Bhatt, N.R.; Shah, M.J. Voglibose: An alpha glucosidase inhibitor. J. Clin. Diagn. Res. 2013, 7, 3023–3027. [Google Scholar] [PubMed]
- Rolfsmeier, M.; Blum, P. Purification and characterization of a maltase from the extremely thermophilic crenarchaeote Sulfolobus solfataricus. J. Bacteriol. 1995, 177, 482–485. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Huh, J.Y.; Nam, S.H.; Kim, D.; Lee, S.B. Synthesis of quercetin-3-O-glucoside from rutin by Penicillium decumbens naringinase. J. Food Sci. 2013, 78, C411–C415. [Google Scholar] [CrossRef]
- Gao, H.; Kawabata, J. α-Glucosidase inhibition of 6-hydroxyflavones. Part 3: Synthesis and evaluation of 2,3,4-trihydroxybenzoyl-containing flavonoid analogs and 6-aminoflavones as α-glucosidase inhibitors. Bioorg. Med. Chem. 2005, 13, 1661–1671. [Google Scholar] [CrossRef] [Green Version]
- Fisman, E.Z.; Tenenbaum, A.; Motro, M.; Adler, Y. Oral antidiabetic therapy in patients with heart disease. A cardiologic standpoint. Herz 2004, 29, 290–298. [Google Scholar] [CrossRef]
- Tillekeratne, L.M.; Sherette, A.; Fulmer, J.A.; Hupe, L.; Hupe, D.; Gabbara, S.; Peliska, J.A.; Hudson, R.A. Differential inhibition of polymerase and strand-transfer activities of HIV-1 reverse transcriptase. Bioorg. Med. Chem. Lett. 2002, 12, 525–528. [Google Scholar] [CrossRef]
- Houck, C.M.; Pear, J.R.; Elliott, R.; Perchorowicz, J.T. Isolation of DNA encoding sucrase genes from Streptococcus salivarius and partial characterization of the enzymes expressed in Escherichia coli. J. Bacteriol. 1987, 169, 3679–3684. [Google Scholar] [CrossRef] [Green Version]
- Park, J.M.; Bong, H.Y.; Jeong, H.I.; Kim, Y.K.; Kim, J.Y.; Kwon, O. Postprandial hypoglycemic effect of mulberry leaf in Goto-Kakizaki rats and counterpart control Wistar rats. Nutr. Res. Pract. 2009, 3, 272–278. [Google Scholar] [CrossRef]
- Fisman, E.Z.; Motro, M.; Tenenbaum, A. Non-insulin antidiabetic therapy in cardiac patients: Current problems and future prospects. Adv. Cardiol. 2008, 45, 154–170. [Google Scholar] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.-T.; Chuang, Y.-H.; Liao, J.-H.; Hsieh, J.-F. Synthesis of 2-(4-hydroxyphenyl)ethyl 3,4,5-Trihydroxybenzoate and Its Inhibitory Effect on Sucrase and Maltase. Processes 2020, 8, 1603. https://doi.org/10.3390/pr8121603
Li W-T, Chuang Y-H, Liao J-H, Hsieh J-F. Synthesis of 2-(4-hydroxyphenyl)ethyl 3,4,5-Trihydroxybenzoate and Its Inhibitory Effect on Sucrase and Maltase. Processes. 2020; 8(12):1603. https://doi.org/10.3390/pr8121603
Chicago/Turabian StyleLi, Wen-Tai, Yu-Hsuan Chuang, Jiahn-Haur Liao, and Jung-Feng Hsieh. 2020. "Synthesis of 2-(4-hydroxyphenyl)ethyl 3,4,5-Trihydroxybenzoate and Its Inhibitory Effect on Sucrase and Maltase" Processes 8, no. 12: 1603. https://doi.org/10.3390/pr8121603
APA StyleLi, W. -T., Chuang, Y. -H., Liao, J. -H., & Hsieh, J. -F. (2020). Synthesis of 2-(4-hydroxyphenyl)ethyl 3,4,5-Trihydroxybenzoate and Its Inhibitory Effect on Sucrase and Maltase. Processes, 8(12), 1603. https://doi.org/10.3390/pr8121603