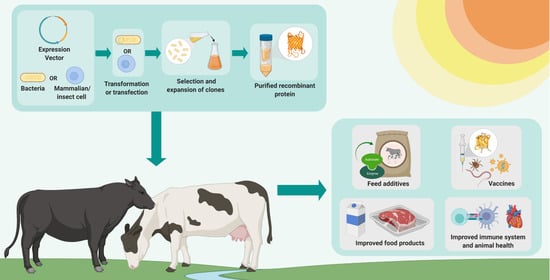
Recombinant Technologies to Improve Ruminant Production Systems: The Past, Present and Future
Abstract
:1. Introduction
2. Ruminants as a Food Source for Humans and Model of Study of Recombinant Proteins
3. Recombinant Hormones for Improving Performance and Fertility
4. Recombinant Proteins for Improving Immune Function
| Product | Protein | Name | Effect | Expression System | Gene | Mode of Use | Purified | Commercially Available | Results | Author |
|---|---|---|---|---|---|---|---|---|---|---|
| Hormone | Bovine Somatotropin | rBST | Increased feed efficiency and production | E. coli K-12 and BL21 | Bovine genome | One or more injections | Ys | Yes | Increased milk production, weight gain in both dairy and beef cattle | [5,20,21,22] |
| Binding protein | Alpha-lactalbumin | alpha-LA | Promote lactose production in dairy cows | Dairy cow | Human alpha-LA | Nuclear transfer of cow embryos | Yes | No | Alpha-LA did not increase lactose concentration in dairy cows | [6] |
| Cytokine | Interleukin-8 | rbIL-8 | Improve immune response in cattle | E. coli BL21 | Bovine IL-8 gene | Intravaginal administration | Yes | No | Recombinant rBIL-8 improved the immune response and milk production in dairy cows | [15,41] |
| Hormone | Growth hormone | SbV | Increased muscle deposition | E. coli BL21 | Bovine genome | Single injection | Yes | Yes | Increased daily gain and muscle size in beef cattle | [21] |
| Hormone | Follicle stimulatory | roFSH | Superovulatory activity | Pichia pastoris | Bovine genome | Single injection | No | Yes | Improve reproductive performance in cattle and sheep | [26] |
| Hormone | Placental Lactogen | rbPL/rbPRL | Improve mammary growth and milk production | E. coli BL21 | Bovine genome | Continuous injections | Yes | Yes | Application of rbPL did not increased milk production in dairy cows | [29] |
| Transport protein | Releasing factor | bGRF | Delivery protein system for ruminants | Bovine leukemia virus | Bovine genome | Transfection | Yes | No | The virus infected bovine cells and released bGRF | [33] |
| Protein | Lysostaphin | rLYS | Protein to improve immune response against metritis in cows | Staphylococcus aureus | Bovine genome | Injection | Yes | Yes | Application of rLYS did not reduce udder infection in dairy cows | [34] |
| Cytokine | Interleukin-2 | rbIL-2 | Improve immune response in cattle | E. coli BL21 | Bovine genome | Intramammary infusion | Yes | No | Application of rbIL-2 was not effective in dairy cows | [35] |
| Cytokine | Tumor necrosis factor | rbTNF | Improve energy metabolism and immune response | B. brevis | Bovine genome | Single injection | Yes | Yes | Reduced insulin resistance, improved immune status in heifers | [39,40] |
| Immune cell | amyloid A3 | M-SAA3 | Stimulate innate immunity and prevent udder infections | E. coli BL21 | Caprine genome | Incubation in mammary cells | Yes | No | Recombinant M-SAA3 reduced numbers of pathogenic bacteria | [42] |
5. Recombinant Vaccines for Improving Immune Response
| Product | Protein | Name | Effect | Expression System | Gene | Mode of Use | Purified | Commercially Available | Results | Author |
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine | Antigen | yidR | Immunity against Klebsiella pneumoniae | E. coli BL21 | yidR | Immunization using purified protein | Yes | No | ~90% of effectiveness in mice | [16] |
| Vaccine | Antigen | Vrec | Recombinant vaccine against botulism | E. coli BL21 | HCBoNT | Immunization using crude extract | No | Yes | Protection for less than 180 days in buffaloes | [45] |
| Vaccine/Protein | Antigen | rBM86 | Vaccine against bovine ticks (R. Boophilus) | Pichia pastoris MB9 | BM86 gene present in ticks | Single injection | Yes | Yes | Provides immune response in domesticated and wild ruminants | [44,57] |
| Vaccine | Antigen | HcGAPDH | Protein against H. contortus parasite | E. coli BL21/B. subtillis | CotB | Single injection | Yes | No | Protective effects against H. contortus in sheep | [64] |
| Vaccine | Antigen/Toxin | D-epsion toxin | Vaccine to reduce enterotoxaemia by clostridium perfringers | E. coli BL21 | ext gene | Immunization using insoluble fraction | No | Yes | It was effective in rabbits and cattle | [65] |
| Vaccine | Antigen/Probiotic | pPG-E20-ctxB | Recombinant vaccine against bovine diarrhea virus | Lactobacillus W56 | V. cholerae OG80 genome | Direct-fed microbe | Yes | No | Provides immune response in mice | [66] |
| Vaccine | Antigen | LKT/PLO | Vaccine against puerperal metritis in dairy cows | E. coli 4612-2 | PLO gene, FimH gene | Subcutaneous injection and intravaginal | Yes | Yes | Increased the immune response increasing lgG titers | [67] |
| Vaccine | Antigen | EhaF | Reduce methanogenesis | E. coli DE3 | KP453861 | Intradermal vaccination | Yes | No | The vaccine did not reduce CH4 emissions in goats | [68] |
| Vaccine/Virus | Recombinant virus | Recombinant capripoxvirus reCapPPR/F | Chimera virus to protect against PPRV and capripoxvirus infections | Lamb testis cells | TK gene from ca | PPRV F gene and Thymidine kinase from capripoxvirus | Yes | No | The chimera virus protected goats against PPRV and caproxvirus | [53] |
| Vaccine/Virus | Recombinant virus | Recombinant new castle virus rNDV_H | Recombinant vaccine against peste de petits ruminant virus (PPRV) | Chicken embryo fibroblasts | Glycoprotein h from PPRV | Subcutaneous injections | Yes | No | Administration of rNDV provided protection against PPRV | [70] |
6. Recombinant Enzymes for Improving Ruminal Fermentation
Xylanases, Beta-Glucanases, and Amylases
7. Recombinant Direct-Fed Microbes
8. Recombinant Technologies to Improve Sustainability of Animal Food Sources
9. Transgenic Animals
10. Gene Editing: An Emergent Technology to Improve Animal Production Systems
| Type | Protein | Name | Effect | Expression System | Gene | Mode of Use | Purified | Commercially Available | Results | Author |
|---|---|---|---|---|---|---|---|---|---|---|
| Enzyme | Protease | ZDP | Zein-degrading protease | Pichia pastoris X-33 | Zeocin gene | Incubation with alpha amylases | Yes | No | Synergistic hydrolysis of starch between ZDP and amylases | [54] |
| Enzyme | Cellulase | pILCT-C | Fungal cellulase in L. lactis for silage inoculants | E. coli V850 | Neocallimastix fungi genome | Inoculation in silage | No | No | Increased NDF hydrolysis in alfalfa samples | [75] |
| Enzyme-like | Swollenin | pPICZalphaA | Disruptive activity towards cellulose | Pichia pastoris X-33 and E. Coli DH5alpha | swoF | Purified Swollenin + fibrolytic enzyme were applied to a diet for in vitro digestibility | Yes | No | Increased in vitro fermentation and acetate concentration | [76] |
| Enzyme-like | Expansin-like protein | BsEXLX1 | Increase cellulose and hemicellulose fermentation | E. coli BL21 | yoaJ | Direct application to the substrate | Yes | No | Synergistic degradation of fiber with fibrolytic enzymes. Increased rumen fermentation in vitro | [77,90] |
| Enzyme | Xylanase | rLexyn11a | Hydrolytic activity towards hemicellulose | Pichia pastoris X-33 | XynR | Direct incubation in the diet for beef cattle | Yes | No | Increased in vitro fermentation and increase acetate and butyrate concentrations | [78] |
| Enzyme | Amylase | Rumistart | Increased hydrolysis of starch in the rumen | Bacillus licheniformis | NA | Direct application to the feed | No | Yes | Recombinant amylase increased feed efficiency and milk production in dairy cows | [79] |
| Enzyme | Celullase- Xylanase | GH10/XYL10A | Increased cellulose and hemicellulose degradation | E. coli BL21 | Aspergillus Niger genome | Direct application to the substrate | Yes | No | Recombinant enzymes increased degradation of straw in vitro and daily gain in sheep | [80,89,134] |
| Enzyme | Laccase | LeLac | Degradation of lignin from lignocellulose | E. coli Bl21 and P. Pastoris | L. edodes AB035409.1 gene | Direct application to the substrate | Yes | No | Increased lignin degradation in straw | [93] |
| Enzyme | xylanase | XOS | Hydrolysis of hemicellulose | Pichia pastoris GS115 | Xyn10CF | Direct incubation in agricultural waste | No | No | Increased hydrolysis of hemicellulose | [135] |
| Enzyme | Cellulase | CMC-1 and EP | Improve fiber fermentation | E. coli BL21 | CMC-1, EP-15 | Hydrolysis of cellulose at rumen conditions | Yes | No | High activity towards cellulose at rumen conditions | [136] |
| Enzyme | Amylase | amyB | Increasing hydrolysis of starch | Bacillus choshinensis | Amybeta | Crude extract obtained by solid-state fermentation | No | No | Increased glucose release from starch | [137] |
| Enzyme | Xylanase | xynS20 | Increase hemicellulose digestibility in the rumen | E. coli BL21 | N. patriciarum genome | Direct application to the substrate | Yes | No | Increased hydrolysis of lignocellulose | [138] |
| Product | Protein | Name | Effect | Expression System | Gene | Mode of Use | Purified | Commercially Available | Results | Author |
|---|---|---|---|---|---|---|---|---|---|---|
| Yeast | Amylase and glycoamlyse | alpha- amylase | Increasing starch fermentation | S. cerevisiae MT8-1 (lithium acetate method) | SBA, SBAI, SBAII, SABIII | Incubation in corn | no | no | Increased fermentation of starch compared to the control | [83] |
| Yeast | Amylase | Y294-Amy | Increase starch hydrolysis and fermentation | S. cerevisiae Y294 and E. coli (lithium acetate method) | apuA, temA, ateG, temG | Direct application to the substrate | Yes | No | Increased starch fermentation | [84] |
| Bacteria | fungal xylanase | xynA | Improving fiber fermentation | E. coli BL21 | pNPXD2 | Direct-fed microbe | Yes | No | Low competitiveness with rumen microbes | [101] |
| Bacteria | Xylanase | xynA | Increase fermentation of hemicellulose | Butyrivibrio fibriosolvens | xynA, pUMSXr | Direct-fed microbe | Yes | No | Low competitiveness with rumen microbes | [103] |
| Bacteria | Cellulase | rLB pM25 | Increase fiber degradation and fermentation in the rumen | Lactobacillus Plantarum | Clostridium thermocellum | Direct-fed microbe | Yes | No | The bacteria were rapidly lost by protozoal predation | [104] |
| Yeast | Cellulase | BGL1 | Recombinant S. cerevisiae with B-glucosidase and Cellulase | E. coli XL1 and S. cerevisiae Y294 | X99228, AB003694 | Hydrolysis of purified cellulose | Yes | Yes | Increased hydrolysis of cellulose | [107] |
| Bacteria | Dehalogenase | pBHf | Prevent fluoroacetate poisoning in ruminants | Butyrivibrio fibriosolvens | M. species | Direct-fed microbe | Yes | Yes | Recombinant bacterium prevented poisoning in sheep | [105,106] |
| Yeast | Amylase | pYDI | Increase starch hydrolysis and fermentation | E. coli Bl21 and S. cerevisiae | Aspergillus niger NRRL334 | Direct incubation in the rumen | Yes | No | Increased starch hydrolysis at rumen conditions | [108] |
| Bacteria | Xylanase | rBTX | Increase hemicellulose digestibility in the rumen | Bacteroides thetaiotaomicron | Prevotella ruminicola genome | Direct-fed microbe | Yes | No | Did not improve fermentation | [133] |
| Type | Protein | Name | Effect | Expression System | Gene | Mode of Use | Purified | Commercially Available | Results | Author |
|---|---|---|---|---|---|---|---|---|---|---|
| Enzyme | Transglutaminase | MTG | Improve meat product quality | Pichia pastoris GS115 | TGase gene from S. fradiae | Direct application in restructured meat | Yes | No | Direct application of MTG increased meat quality | [113] |
| Protein/ bacteria | Antifreeze protein | rAFP expressed by L. lactis | Improve cryogenic preservation of meat | Lactobacillus Acidophilus, Lactoccocus lactis | SlpA from L. Acidophilus, | Direct application of lyophilized crude extract on meat and dough | No | No | Increased juiciness and reduced protein loss in frozen meat. Improved fermentation of dough | [114] |
| Protein | Colicin | ColM | Antibacterial activity for meat and food | Nicotiana Benthamiana | Colicin gene from E. coli | Direct application on meat | Yes | No | Application of ColM reduced E. coli counts on fresh steak meat | [115,116] |
| Enzyme | Chymosin | RLC | Improve milk clotting for cheese production | E. coli | Lamb gene | Direct incubation on milk for cheese production | Yes | Yes | Improved cheese production compared to the control | [109,117] |
| Probiotic | Monellin | MNEI | Sweetener from cheese whey | Lactococcus lactis/E. coli | MNEI gene | Direct incubation of L. lactis on cheese whey | Yes | No | This strategy valorized dairy effluents like cheese whey to produce Sweetener and probiotics | [111] |
| Enzyme | Galactosidase | bgaB | Improve Lactose hydrolysis | Bacillus subtilis | Bacillus Stearothermophilus gene | Direct incubation on whole-milk for lactose-free milk production | Yes | Yes | Improved hydrolysis of lactose in milk | [112,139] |
| Probiotic | Enzyme | RD-534 | Improve exopolysaccharides in yogurt | Streptococcus thermophilus RD534 | S. thermophilus DGCC7710 | Direct incubation on milk for yogurt production | Yes | No | Addition of RD-534-S1 increased production of exopolysaccharides | [140] |
| Enzyme | Transglutaminase | TGZo | Food enhancer to produce yogurt | Pichia pastoris GS115 | TGZo gene from Corn | Incubation for yogurt production | Yes | No | TGzo increased consistency, cohesiveness and viscosity in yogurt | [141] |
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Balehegn, M.; Duncan, A.; Tolera, A.; Ayantunde, A.A.; Issa, S.; Karimou, M.; Zampaligré, N.; André, K.; Gnanda, I.; Varijakshapanicker, P. Improving adoption of technologies and interventions for increasing supply of quality livestock feed in low- and middle-income countries. Glob. Food Secur. 2020, 26, 100372. [Google Scholar] [CrossRef]
- Adesogan, A.T.; Havelaar, A.H.; McKune, S.L.; Eilittä, M.; Dahl, G.E. Animal source foods: Sustainability problem or malnutrition and sustainability solution? Perspective matters. Glob. Food Secur. 2020, 25. [Google Scholar] [CrossRef]
- Adesogan, A.T.; Arriola, K.G.; Jiang, Y.; Oyebade, A.; Paula, E.M.; Pech-Cervantes, A.A.; Romero, J.J.; Ferraretto, L.F.; Vyas, D. Symposium review: Technologies for improving fiber utilization. J. Dairy Sci. 2019, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandler, R. The ethics of genetic engineering and gene drives in conservation. Conserv. Biol. 2020, 34, 378–385. [Google Scholar] [CrossRef]
- Bauman, D.E. Bovine Somatotropin: Review of an Emerging Animal Technology. J. Dairy Sci. 1992, 75, 3432–3451. [Google Scholar] [CrossRef]
- Wang, J.; Yang, P.; Tang, B.; Sun, X.; Zhang, R.; Guo, C.; Gong, G.; Liu, Y.; Li, R.; Zhang, L.; et al. Expression and characterization of bioactive recombinant human α-lactalbumin in the milk of transgenic cloned cows. J. Dairy Sci. 2008, 91, 4466–4476. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.X.; Yang, Y.; de Souza Meira, E.B.; do Carmo Silva, J.; Bicalho, R.C. Development and evaluation of a new recombinant protein vaccine (YidR) against Klebsiella pneumoniae infection. Vaccine 2020, 38, 4640–4648. [Google Scholar] [CrossRef]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Gerber, P.J.; Mottet, A.; Opio, C.I.; Falcucci, A.; Teillard, F. Environmental impacts of beef production: Review of challenges and perspectives for durability. Meat Sci. 2015, 109, 2–12. [Google Scholar] [CrossRef]
- Forsberg, C.W.; Crosby, B.; Thomas, D.Y. Potential for Manipulation of the Rumen Fermentation. Biotechnol. Res. Innov. 1986, 63, 310–325. [Google Scholar]
- Rischer, H.; Szilvay, G.R.; Oksman-Caldentey, K.M. Cellular agriculture—Industrial biotechnology for food and materials. Curr. Opin. Biotechnol. 2020, 61, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Ripple, W.J.; Smith, P.; Haberl, H.; Montzka, S.A.; McAlpine, C.; Boucher, D.H. Ruminants, climate change and climate policy. Nat. Clim. Chang. 2014, 4, 2–5. [Google Scholar] [CrossRef]
- Hackmann, T.J.; Firkins, J.L. Maximizing efficiency of rumen microbial protein production. Front. Microbiol. 2015, 6, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouwman, A.C.; Visker, M.H.P.W.; van Arendonk, J.A.M.; Bovenhuis, H. Fine mapping of a quantitative trait locus for bovine milk fat composition on Bos taurus autosome 19. J. Dairy Sci. 2014, 97, 1139–1149. [Google Scholar] [CrossRef] [Green Version]
- Zinicola, M.; Bicalho, M.L.S.; Santin, T.; Marques, E.C.; Bisinotto, R.S.; Bicalho, R.C. Effects of recombinant bovine interleukin-8 (rbIL-8) treatment on health, metabolism, and lactation performance in Holstein cattle II: Postpartum uterine health, ketosis, and milk production. J. Dairy Sci. 2019, 102, 10316–10328. [Google Scholar] [CrossRef]
- Meira, E.B.S.; Ellington-Lawrence, R.D.; Silva, J.C.C.; Higgins, C.H.; Linwood, R.; Rodrigues, M.X.; Bringhenti, L.; Korzec, H.; Yang, Y.; Zinicola, M.; et al. Recombinant protein subunit vaccine reduces puerperal metritis incidence and modulates the genital tract microbiome. J. Dairy Sci. 2020, 103, 7364–7376. [Google Scholar] [CrossRef]
- Hartman, S.; Horner, W.; Jackson, C.; Kovak, E.; Velan, V. Streamlining USDA Regulation of Gene Editing to Benefit US Agriculture. J. Sci. Policy Gov. 2020, 17. [Google Scholar] [CrossRef]
- Berg, P.; Singer, M. The recombinant DNA controversy: Twenty years later. Bio/Technology 1995, 13, 1132–1134. [Google Scholar] [CrossRef]
- Lamas, A.; Regal, P.; Vázquez, B.; Miranda, J.M.; Cepeda, A.; Franco, C.M. Tracing recombinant bovine somatotropin ab(use) through transcriptomics: The potential of bovine somatic cells in a multi-dose longitudinal study. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Dohoo, I.R.; DesCôteaux, L.; Leslie, K.; Fredeen, A.; Shewfelt, W.; Preston, A.; Dowling, P. A meta-analysis review of the effects of recombinant bovine somatotropin 2. Effects on animal health, reproductive performance, and culling. Can. J. Vet. Res. 2003, 67, 252–264. [Google Scholar]
- Elsasser, T.H.; Rumsey, T.S.; Kahl, S.; Czerwinski, S.M.; Moseleyt, W.M.; Ono, Y.; Solomon, M.B.; Harris, F.; Fagan, J.M. Effects of Synovex-S® and Recombinant Bovine Growth Hormone (Somavubove®) on Growth Responses of Steers: III. Muscle Growth and Protein Responses. J. Anim. Sci. 1998, 76, 2346–2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohoo, I.R.; Leslie, K.; DesCôteaux, L.; Fredeen, A.; Dowling, P.; Preston, A.; Shewfelt, W. A meta-analysis review of the effects of recombinant bovine somatotropin 1. Methodology and effects on production. Can. J. Vet. Res. 2003, 67, 241–251. [Google Scholar] [PubMed]
- Silva, P.R.B.; Machado, K.S.; Da Silva, D.N.L.; Moraes, J.G.N.; Keisler, D.H.; Chebel, R.C. Effects of recombinant bovine somatotropin during the periparturient period on innate and adaptive immune responses, systemic inflammation, and metabolism of dairy cows. J. Dairy Sci. 2015, 98, 4449–4464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, T.E.; Boime, I. The Expanding Role of Recombinant Gonadotropins in Assisted Reproduction. Reprod. Domest. Anim. 2008, 43, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Hesser, M.W.; Morris, J.C.; Gibbons, J.R. Advances in Recombinant Gonadotropin Production for Use in Bovine Superovulation. Reprod. Domest. Anim. 2011, 46, 933–942. [Google Scholar] [CrossRef]
- Sanderson, N.; Martinez, M. A single administration of a long-acting recombinant ovine FSH (roFSH) for cattle superovulation. Theriogenology 2020, 154, 66–72. [Google Scholar] [CrossRef]
- Fidler, A.E.; Lun, S.; Young, W.; McNatty, K.P. Expression and secretion of a biologically active glycoprotein hormone, ovine follicle stimulating hormone, by Pichia pastoris. J. Mol. Endocrinol. 1998, 21, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Chupin, D.; Procureur, R. Efficiency of pituitary extracts (FSH) for induction of superovulation in cattle. Anim. Reprod. Sci. 1983, 6, 11–23. [Google Scholar] [CrossRef]
- Byatt, J.C.; Sorbet, R.H.; Eppard, P.J.; Curran, T.L.; Curran, D.F.; Collier, R.J. The Effect of Recombinant Bovine Placental Lactogen on Induced Lactation in Dairy Heifers. J. Dairy Sci. 1997, 80, 496–503. [Google Scholar] [CrossRef]
- Alvarez-Oxiley, A.V.; De Sousa, N.M.; Beckers, J.F. Native and recombinant bovine placental lactogens. Reprod. Biol. 2008, 8, 85–106. [Google Scholar] [CrossRef]
- Seow, Y.; Wood, M.J. Biological gene delivery vehicles: Beyond viral vectors. Mol. Ther. 2009, 17, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Neumann, G.; Kawaoka, Y.; Hobom, G.; Webster, R.G. A DNA transfection system for generation of influenza a virus from eight plasmids. Proc. Natl. Acad. Sci. USA 2000, 97, 6108–6113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehigh, C.S.; Elias, V.D.; Mehigh, R.J.; Helferich, W.G.; Tucker, H.A. Development of a recombinant bovine leukemia virus vector for delivery of a synthetic bovine growth hormone-releasing factor gene into bovine cells. J. Anim. Sci. 1993, 71, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Oldham, E.R.; Daley, M.J. Lysostaphin: Use of a Recombinant Bactericidal Enzyme as a Mastitis Therapeutic. J. Dairy Sci. 1991, 74, 4175–4182. [Google Scholar] [CrossRef]
- Hogan, J.S.; Smith, K.L.; Todhunter, D.A.; Schoenberger, P.S.; Shuster, D. Efficacy of Recombinant Bovine Interleukin-2 as an Adjunct to Dry Cow Therapy. J. Dairy Sci. 1995, 78, 1062–1067. [Google Scholar] [CrossRef]
- De Vliegher, S.; Fox, L.K.; Piepers, S.; McDougall, S.; Barkema, H.W. Invited review: Mastitis in dairy heifers: Nature of the disease, potential impact, prevention, and control. J. Dairy Sci. 2012, 95, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Rollin, E.; Dhuyvetter, K.C.; Overton, M.W. The cost of clinical mastitis in the first 30 days of lactation: An economic modeling tool. Prev. Vet. Med. 2015, 122, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Wall, R.J.; Powell, A.M.; Paape, M.J.; Kerr, D.E.; Bannerman, D.D.; Pursel, V.G.; Wells, K.D.; Talbot, N.; Hawk, H.W. Genetically enhanced cows resist intramammary Staphylococcus aureus infection. Nat. Biotechnol. 2005, 23, 445–451. [Google Scholar] [CrossRef]
- Kushibiki, S.; Hodate, K.; Shingu, H.; Obara, Y.; Touno, E.; Shinoda, M.; Yokomizo, Y. Metabolic and lactational responses during recombinant bovine tumor necrosis factor-α treatment in lactating cows. J. Dairy Sci. 2003, 86, 819–827. [Google Scholar] [CrossRef] [Green Version]
- Bradford, B.J.; Mamedova, L.K.; Minton, J.E.; Drouillard, J.S.; Johnson, B.J. Daily injection of tumor necrosis factor-α increases hepatic triglycerides and alters transcript abundance of metabolic genes in lactating dairy cattle. J. Nutr. 2009, 139, 1451–1456. [Google Scholar] [CrossRef] [Green Version]
- Bicalho, M.L.S.; Zinicola, M.; Machado, V.S.; Lima, F.S.; Teixeira, A.G.V.; Narbus, C.; Xavier, M.R.; Higgins, H.; Bicalho, R.C. Effects of recombinant bovine interleukin-8 (rbIL-8) treatment on health, metabolism, and lactation performance in Holstein cattle I: Production and functional characterization of rbIL-8 in vitro and in vivo. J. Dairy Sci. 2019, 102, 10304–10315. [Google Scholar] [CrossRef] [PubMed]
- Parés, S.; Fàbregas, F.; Bach, À.; Garcia-Fruitós, E.; de Prado, A.; Arís, A. Short communication: Recombinant mammary serum amyloid A3 as a potential strategy for preventing intramammary infections in dairy cows at dryoff. J. Dairy Sci. 2020, 103, 3615–3621. [Google Scholar] [CrossRef] [PubMed]
- Clay, N.; Garnett, T.; Lorimer, J. Dairy intensification: Drivers, impacts and alternatives. Ambio 2020, 49, 35–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canales, M.; Enríquez, A.; Ramos, E.; Cabrera, D.; Dandie, H.; Soto, A.; Falcón, V.; Rodríguez, M.; De la Fuente, J. Large-scale production in Pichia pastoris of the recombinant vaccine GavacTM against cattle tick. Vaccine 1997, 15, 414–422. [Google Scholar] [CrossRef]
- Otaka, D.Y.; Barbosa, J.D.; de Souza, L.A.; Moreira, C.; Ferreira, M.R.A.; Donassolo, R.A.; Conceição, F.R.; Salvarani, F.M. Recombinant vaccine against botulism in buffaloes: Evaluation of the humoral immune response over 12 months. Anaerobe 2020, 63, 102201. [Google Scholar] [CrossRef] [PubMed]
- Kaddar, M. Global vaccine market features and trends. Work. Bus. Model. Sustain. Influ. Vaccine Manuf. 2013, 1–38. [Google Scholar]
- Jorge, S.; Dellagostin, O.A. The development of veterinary vaccines: A review of traditional methods and modern biotechnology approaches. Biotechnol. Res. Innov. 2017, 1, 6–13. [Google Scholar] [CrossRef]
- Correia, B.E.; Bates, J.T.; Loomis, R.J.; Baneyx, G.; Carrico, C.; Jardine, J.G.; Rupert, P.; Correnti, C.; Kalyuzhniy, O.; Vittal, V.; et al. Proof of principle for epitope-focused vaccine design. Nature 2014, 507, 201–206. [Google Scholar] [CrossRef]
- Miles, A.P.; McClellan, H.A.; Rausch, K.M.; Zhu, D.; Whitmore, M.D.; Singh, S.; Martin, L.B.; Wu, Y.; Giersing, B.K.; Stowers, A.W.; et al. Montanide® ISA 720 vaccines: Quality control of emulsions, stability of formulated antigens, and comparative immunogenicity of vaccine formulations. Vaccine 2005, 23, 2530–2539. [Google Scholar] [CrossRef]
- Garçon, N.; Wettendorff, M.; Mechelen, M. Van Role of AS04 in human papillomavirus vaccine: Mode of action and clinical profile. Expert Opin. Biol. Ther. 2011, 11, 667–677. [Google Scholar] [CrossRef]
- Marchioro, S.B.; Maes, D.; Flahou, B.; Pasmans, F.; Del Pozo Sacristán, R.; Vranckx, K.; Melkebeek, V.; Cox, E.; Wuyts, N.; Haesebrouck, F. Local and systemic immune responses in pigs intramuscularly injected with an inactivated Mycoplasma hyopneumoniae vaccine. Vaccine 2013, 31, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Odir, A.D.; Grassmann, A.A.; Hartwig, D.D.; Félix, S.R.; Da Silva, É.F.; McBride, A.J.A. Recombinant vaccines against leptospirosis. Hum. Vaccines 2011, 7, 1215–1224. [Google Scholar] [CrossRef]
- Berhe, G.; Minet, C.; Le Goff, C.; Barrett, T.; Ngangnou, A.; Grillet, C.; Libeau, G.; Fleming, M.; Black, D.N.; Diallo, A. Development of a Dual Recombinant Vaccine to Protect Small Ruminants against Peste-des-Petits-Ruminants Virus and Capripoxvirus Infections. J. Virol. 2003, 77, 1571–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Liu, C.; Qu, M.; Pan, K.; Ouyang, K.; Song, X.; Zhao, X. Characterization of a recombinant zein-degrading protease from Zea mays by Pichia pastoris and its effects on enzymatic hydrolysis of corn starch. Int. J. Biol. Macromol. 2020, 164, 3287–3293. [Google Scholar] [CrossRef]
- Ghosh, S.; Nagar, G. Problem of ticks and tick-borne diseases in india with special emphasis on progress in tick control research: A review. J. Vector Borne Dis. 2014, 51, 259–270. [Google Scholar]
- Eshghi, A.; Cullen, P.A.; Cowen, L.; Zuerner, R.L.; Cameron, C.E. Global proteome analysis of Leptospira interrogans. J. Proteome Res. 2009, 8, 4564–4578. [Google Scholar] [CrossRef] [Green Version]
- Bastos, R.G.; Ueti, M.W.; Knowles, D.P.; Scoles, G.A. The Rhipicephalus (Boophilus) microplus Bm86 gene plays a critical role in the fitness of ticks fed on cattle during acute Babesia bovis infection. Parasites Vectors 2010, 3, 111. [Google Scholar] [CrossRef] [Green Version]
- Morgan, J.A.T.; Corley, S.W.; Jackson, L.A.; Lew-Tabor, A.E.; Moolhuijzen, P.M.; Jonsson, N.N. Identification of a mutation in the para-sodium channel gene of the cattle tick Rhipicephalus (Boophilus) microplus associated with resistance to synthetic pyrethroid acaricides. Int. J. Parasitol. 2009, 39, 775–779. [Google Scholar] [CrossRef] [Green Version]
- Perez-Cogollo, L.C.; Rodriguez-Vivas, R.I.; Ramirez-Cruz, G.T.; Miller, R.J. First report of the cattle tick Rhipicephalus microplus resistant to ivermectin in Mexico. Vet. Parasitol. 2010, 168, 165–169. [Google Scholar] [CrossRef]
- Ehsan, M.; Hu, R.S.; Liang, Q.L.; Hou, J.L.; Song, X.; Yan, R.; Zhu, X.Q.; Li, X. Advances in the development of anti-haemonchus contortus vaccines: Challenges, opportunities, and perspectives. Vaccines 2020, 8, 555. [Google Scholar] [CrossRef]
- Estrada-Reyes, Z.M.; Tsukahara, Y.; Amadeu, R.R.; Goetsch, A.L.; Gipson, T.A.; Sahlu, T.; Puchala, R.; Wang, Z.; Hart, S.P.; Mateescu, R.G. Signatures of selection for resistance to Haemonchus contortus in sheep and goats. BMC Genom. 2019, 20, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cachat, E.; Newlands, G.F.J.; Ekoja, S.E.; McAllister, H.; Smith, W.D. Attempts to immunize sheep against Haemonchus contortus using a cocktail of recombinant proteases derived from the protective antigen, H-gal-GP. Parasite Immunol. 2010, 32, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.; Antonopoulos, A.; Haslam, S.M.; Dicker, A.J.; Mcneilly, T.N.; Johnston, S.L.; Dell, A.; Knox, D.P.; Britton, C. Novel expression of Haemonchus contortus vaccine candidate aminopeptidase H11 using the free-living nematode Caenorhabditis elegans. Vet. Res. 2013, 44, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhang, G.; Wu, J.; Chen, X.; Tong, D.; Yang, Y.; Shi, H.; Yao, C.; Zhuang, L.; Wang, J.; et al. Recombinant HcGAPDH Protein Expressed on Probiotic Bacillus subtilis Spores Protects Sheep from Haemonchus contortus Infection by Inducing both Humoral and Cell- Mediated Responses. Msystems 2020, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lobato, F.C.F.; Lima, C.G.R.D.; Assis, R.A.; Pires, P.S.; Silva, R.O.S.; Salvarani, F.M.; Carmo, A.O.; Contigli, C.; Kalapothakis, E. Potency against enterotoxemia of a recombinant Clostridium perfringens type D epsilon toxoid in ruminants. Vaccine 2010, 28, 6125–6127. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Huang, X.; Li, H.; Zheng, D.; Wang, L.; Qiao, X.; Jiang, Y.; Cui, W.; Tang, L.; Li, Y.; et al. Immunogenicity evaluation of recombinant Lactobacillus casei W56 expressing bovine viral diarrhea virus E2 protein in conjunction with cholera toxin B subunit as an adjuvant. Microb. Cell Fact. 2020, 19, 1–20. [Google Scholar] [CrossRef]
- Machado, V.S.; De Souza Bicalho, M.L.; De Souza Meira, E.B.; Rossi, R.; Ribeiro, B.L.; Lima, S.; Santos, T.; Kussler, A.; Foditsch, C.; Ganda, E.K.; et al. Subcutaneous immunization with inactivated bacterial components and purified protein of Escherichia coli, Fusobacterium necrophorumand Trueperella pyogenes prevents puerperal metritis in holstein dairy cows. PLoS ONE 2014, 9, e91734. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, X.; Xue, B.; Peng, Q.; Wang, Z.; Yan, T.; Wang, L. Immunization against rumen methanogenesis by vaccination with a new recombinant protein. PLoS ONE 2015, 10, e0140086. [Google Scholar] [CrossRef] [Green Version]
- Balamurugan, V.; Hemadri, D.; Gajendragad, M.R.; Singh, R.K.; Rahman, H. Diagnosis and control of peste des petits ruminants: A comprehensive review. VirusDisease 2014, 25, 39–56. [Google Scholar] [CrossRef] [Green Version]
- Murr, M.; Hoffmann, B.; Grund, C.; Römer-Oberdörfer, A.; Mettenleiter, T.C. A novel recombinant Newcastle disease virus vectored DIVA vaccine against peste des petits ruminants in goats. Vaccines 2020, 8, 205. [Google Scholar] [CrossRef]
- Adesogan, A.T.; Ma, Z.X.; Romero, J.J.; Arriola, K.G. Ruminant Nutrition Symposium: Improving cell wall digestion and animal performance with fibrolytic enzymes. J. Anim. Sci. 2014, 92, 1317–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arriola, K.G.; Oliveira, A.S.; Ma, Z.X.; Lean, I.J.; Giurcanu, M.C.; Adesogan, A.T. A meta-analysis on the effect of dietary application of exogenous fibrolytic enzymes on the performance of dairy cows. J. Dairy Sci. 2017, 100, 4513–4527. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.J.; Zarate, M.A.; Arriola, K.G.; Gonzalez, C.F.; Silva-Sanchez, C.; Staples, C.R.; Adesogan, A.T. Screening exogenous fibrolytic enzyme preparations for improved in vitro digestibility of bermudagrass haylage. J. Dairy Sci. 2015, 98, 2555–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meale, S.J.; Beauchemin, K.A.; Hristov, A.N.; Chaves, A.V.; McAllister, T.A. BOARD-INVITED REVIEW: Opportunities and challenges in using exogenous enzymes to improve ruminant production. J. Anim. Sci. 2014, 92, 427–442. [Google Scholar] [CrossRef] [Green Version]
- Ozkose, E.; Akyol, I.; Kar, B.; Comlekcioglu, U.; Ekinci, M.S. Expression of fungal cellulase gene in Lactococcus lactis to construct novel recombinant silage inoculants. Folia Microbiol. 2009, 54, 335–342. [Google Scholar] [CrossRef]
- Li, L.; Qu, M.; Liu, C.; Xu, L.; Pan, K.; OuYang, K.; Song, X.; Li, Y.; Liang, H.; Chen, Z.; et al. Effects of recombinant swollenin on the enzymatic hydrolysis, rumen fermentation, and rumen microbiota during in vitro incubation of agricultural straws. Int. J. Biol. Macromol. 2019, 122, 348–358. [Google Scholar] [CrossRef]
- Pech-Cervantes, A.A.; Muhammad, I.; Ogunade, I.M.; Jiang, Y.; Kim, D.H.; Gonzalez, C.F.; Hackmann, T.J.; Oliveira, A.S.; Vyas, D.; Adesogan, A.T. Exogenous fibrolytic enzymes and recombinant bacterial expansins synergistically improve hydrolysis and in vitro digestibility of bermudagrass haylage. J. Dairy Sci. 2019, 102, 8059–8073. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, K.; Liu, C.; Qu, M.; OuYang, K.; Song, X.; Zhao, X. Recombinant Lentinula edodes xylanase improved the hydrolysis and in vitro ruminal fermentation of soybean straw by changing its fiber structure. Int. J. Biol. Macromol. 2020, 151, 286–292. [Google Scholar] [CrossRef]
- Andreazzi, A.S.R.; Pereira, M.N.; Reis, R.B.; Pereira, R.A.N.; Morais Júnior, N.N.; Acedo, T.S.; Hermes, R.G.; Cortinhas, C.S. Effect of exogenous amylase on lactation performance of dairy cows fed a high-starch diet. J. Dairy Sci. 2018, 101, 7199–7207. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, G.O.; Gruninger, R.J.; Jones, D.R.; Beauchemin, K.A.; Yang, W.Z.; Wang, Y.; Abbott, D.W.; Tsang, A.; McAllister, T.A. Effect of ammonia fiber expansion-treated wheat straw and a recombinant fibrolytic enzyme on rumen microbiota and fermentation parameters, total tract digestibility, and performance of lambs. J. Anim. Sci. 2020, 98, skaa116. [Google Scholar] [CrossRef]
- Ravindran, V.; Son, J.-H. Feed enzyme technology: Present status and future developments. Recent Pat. Food Nutr. Agric. 2011, 3, 102–109. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yin, J.; Wang, L.; Yu, B.; Chen, D. Functional characterisation of a recombinant xylanase from Pichia pastoris and effect of the enzyme on nutrient digestibility in weaned pigs. Br. J. Nutr. 2010, 103, 1507–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamakawa, S.I.; Yamada, R.; Tanaka, T.; Ogino, C.; Kondo, A. Repeated fermentation from raw starch using Saccharomyces cerevisiae displaying both glucoamylase and α-amylase. Enzym. Microb. Technol. 2012, 50, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Cripwell, R.A.; Rose, S.H.; Viljoen-bloom, M.; Zyl, W.H. Van Improved raw starch amylase production by Saccharomyces cerevisiae using codon optimisation strategies. FEMS Yeast Res. 2019, 19, foy127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, H.; Bing, Y.; Keying, Z.; Xuemei, D.; Daiwen, C. Expression of a Trichoderma reesei β-xylanase gene in Escherichia coli and activity of the enzyme on fiber-bound substrates. Protein Expr. Purif. 2009, 67, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Graciano, L.; Corrêa, J.M.; Vieira, F.G.N.; Bosetto, A.; Loth, E.A.; Kadowaki, M.K.; Gandra, R.F.; de Cássia Garcia Simão, R. Cloning and Expression of the xynA1 Gene Encoding a Xylanase of the GH10 Group in Caulobacter crescentus. Appl. Biochem. Biotechnol. 2015, 175, 3915–3929. [Google Scholar] [CrossRef]
- Pontonio, E.; Mahony, J.; Di Cagno, R.; O’Connell Motherway, M.; Lugli, G.A.; O’Callaghan, A.; De Angelis, M.; Ventura, M.; Gobbetti, M.; van Sinderen, D. Cloning, expression and characterization of a β-d-xylosidase from Lactobacillus rossiae DSM 15814T. Microb. Cell Fact. 2016, 15, 72. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Riaz, S.; Jamil, A. Molecular cloning of fungal xylanases: An overview. Appl. Microbiol. Biotechnol. 2009, 84, 19–35. [Google Scholar] [CrossRef]
- Badhan, A.; Wang, Y.; Gruninger, R.; Patton, D.; Powlowski, J.; Tsang, A.; McAllister, T. Formulation of enzyme blends to maximize the hydrolysis of alkaline peroxide pretreated alfalfa hay and barley straw by rumen enzymes and commercial cellulases. BMC Biotechnol. 2014, 14, 31. [Google Scholar] [CrossRef] [Green Version]
- Pech-Cervantes, A.A.; Ogunade, I.M.; Jiang, Y.; Irfan, M.; Arriola, K.G.; Amaro, F.X.; Gonzalez, C.F.; DiLorenzo, N.; Bromfield, J.J.; Vyas, D.; et al. An expansin-like protein expands forage cell walls and synergistically increases hydrolysis, digestibility and fermentation of livestock feeds by fibrolytic enzymes. PLoS ONE 2019, 14, e0224381. [Google Scholar] [CrossRef] [Green Version]
- Ran, T.; Saleem, A.M.; Shen, Y.; Ribeiro, G.O.; Beauchemin, K.A.; Tsang, A.; Yang, W.; McAllister, T.A. Effects of a recombinant fibrolytic enzyme on fiber digestion, ruminal fermentation, nitrogen balance, and total tract digestibility of heifers fed a high forage diet. J. Anim. Sci. 2019, 97, 3578–3587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Liu, C.; Ma, Y.; Hong, J.; Zhang, M. Heterologous expression and functional characterization of a novel cellulose-disruptive protein Le EXP2 from Lycopersicum esculentum. J. Biotechnol. 2014, 186, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, W.; Qu, M.; Pan, K.; Zhao, X. Heterologous Expression of Laccase from Lentinula edodes in Pichia pastoris and Its Application in Degrading Rape Straw. Front. Microbiol. 2020, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.K.; Stern, M.D. Influence of direct-fed microbials on ruminal microbial fermentation and performance of ruminants—A Review. Asian-Australas. J. Anim. Sci. 1995, 8, 533–555. [Google Scholar] [CrossRef]
- Peng, H.; Wang, J.Q.; Kang, H.Y.; Dong, S.H.; Sun, P.; Bu, D.P.; Zhou, L.Y. Effect of feeding Bacillus subtilis natto fermentation product on milk production and composition, blood metabolites and rumen fermentation in early lactation dairy cows. J. Anim. Physiol. Anim. Nutr. 2012, 96, 506–512. [Google Scholar] [CrossRef]
- Xu, H.; Huang, W.; Hou, Q.; Kwok, L.; Sun, Z.; Ma, H.; Zhao, F.; Lee, Y.; Zhang, H. The effects of probiotics administration on the milk production, milk components and fecal bacteria microbiota of dairy cows. Sci. Bull. 2017, 62, 767–774. [Google Scholar] [CrossRef] [Green Version]
- Adeyemi, J.A.; Harmon, D.L.; Compart, D.M.P.; Ogunade, I.M. Effects of a blend of Saccharomyces cerevisiae-based direct-fed microbial and fermentation products in the diet of newly weaned beef steers: Growth performance, whole-blood immune gene expression, serum biochemistry, and plasma metabolome1. J. Anim. Sci. 2019, 97, 4657–4667. [Google Scholar] [CrossRef]
- Broadway, P.; Carroll, J.; Sanchez, N. Live Yeast and Yeast Cell Wall Supplements Enhance Immune Function and Performance in Food-Producing Livestock: A Review. Microorganisms 2015, 3, 417–427. [Google Scholar] [CrossRef]
- Luan, S.; Duersteler, M.; Galbraith, E.A.; Cardoso, F.C. Effects of direct-fed Bacillus pumilus 8G-134 on feed intake, milk yield, milk composition, feed conversion, and health condition of pre- and postpartum Holstein cows. J. Dairy Sci. 2015, 98, 6423–6432. [Google Scholar] [CrossRef] [Green Version]
- Utt, E.A.; Eddy, C.K.; Keshav, K.F.; Ingram, L. Sequencing and expression of the Butyrivibrio fibriosolvens xylB Gene Encoding a Novel Bifunctional Protein with P-D-Xylosidase Il l. Appl. Environ. Micro. 1991, 57, 1227–1234. [Google Scholar] [CrossRef] [Green Version]
- Xue, G.P.; Johnson, J.S.; Bransgrove, K.L.; Gregg, K.; Beard, C.E.; Dalrymple, B.P.; Gobius, K.S.; Aylward, J.H. Improvement of expression and secretion of a fungal xylanase in the rumen bacterium Butyrivibrio fibrisolvens OB156 by manipulation of promoter and signal sequences. J. Biotechnol. 1997, 54, 139–148. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yamamoto, M. Factors that limit maintenance of recombinant rumen bacterium in sheep rumen. Anim. Sci. J. 2002, 73, 131–136. [Google Scholar] [CrossRef]
- Krause, D.O.; Bunch, R.J.; Dalrymple, B.D.; Gobius, K.S.; Smith, W.J.M.; Xue, G.P.; McSweeney, C.S. Expression of a modified Neocallimastix patriciarum xylanase in Butyrivibrio fibrisolvens digests more fibre but cannot effectively compete with highly fibrolytic bacteria in the rumen. J. Appl. Microbiol. 2001, 90, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.; Hazlewood, G.; Gilbert, H.; Donnell, A.G.O. Planfarum are rapidly lost from the rumen by protozoal predation. J. Appl. Bacteriol. 1994, 76, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Gregg, K.; Hamdorf, B.; Henderson, K.; Kopecny, J.; Wong, C. Genetically modified ruminal bacteria protect sheep from fluoroacetate poisoning. Appl. Environ. Microbiol. 1998, 64, 3496–3498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregg, K.; Cooper, C.L.; Schafer, D.J.; Sharpe, H.; Beard, C.E.; Allen, G.; Xu, J. Detoxification of the Plant Toxin Fluoroacetate by a Genetically Modified Rumen Bacterium. Bio/Technology 1994, 12, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Den Haan, R.; Rose, S.H.; Lynd, L.R.; van Zyl, W.H. Hydrolysis and fermentation of amorphous cellulose by recombinant Saccharomyces cerevisiae. Metab. Eng. 2007, 9, 87–94. [Google Scholar] [CrossRef]
- Selwal, K.K.; Li, Y.; Yu, Z. Development of arming yeast with amylase enzyme from Aspergillus niger as a model for delivery of enzyme. Innov. Rom. Food Biotech. 2020, 18, 24–36. [Google Scholar]
- Rogelj, I.; Perko, B.; Francky, A.; Penca, V.; Pungerčar, J. Recombinant lamb chymosin as an alternative coagulating enzyme in cheese production. J. Dairy Sci. 2001, 84, 1020–1026. [Google Scholar] [CrossRef]
- Synowiecki, J.; Grzybowska, B.; Zdziebło, A. Sources, properties and suitability of new thermostable enzymes in food processing. Crit. Rev. Food Sci. Nutr. 2006, 46, 197–205. [Google Scholar] [CrossRef]
- Boumaiza, M.; Colarusso, A.; Parrilli, E.; Garcia-Fruitós, E.; Casillo, A.; Arís, A.; Corsaro, M.M.; Picone, D.; Leone, S.; Tutino, M.L. Getting value from the waste: Recombinant production of a sweet protein by Lactococcus lactis grown on cheese whey. Microb. Cell Fact. 2018, 17, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Chen, H.; Xia, Y.; Yang, J.; Zhao, J.; Tian, F.; Zhang, H.P.; Zhang, H. Immobilization of recombinant thermostable β-galactosidase from Bacillus stearothermophilus for lactose hydrolysis in milk. J. Dairy Sci. 2009, 92, 491–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Zhang, Y. Expression of recombinant transglutaminase gene in Pichia pastoris and its uses in restructured meat products. Food Chem. 2019, 291, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.M.; Kao, B.Y.; Peng, H.J. Production of a recombinant Type 1 antifreeze protein analogue by L. lactis and its applications on frozen meat ar frozen dough. J. Agric. Food Chem. 2009, 57, 6216–6223. [Google Scholar] [CrossRef]
- Stephan, A.; Hahn-Löbmann, S.; Rosche, F.; Buchholz, M.; Giritch, A.; Gleba, Y. Simple purification of Nicotiana benthamiana-produced recombinant colicins: High-yield recovery of purified proteins with minimum alkaloid content supports the suitability of the host for manufacturing food additives. Int. J. Mol. Sci. 2018, 19, 95. [Google Scholar] [CrossRef] [Green Version]
- Schulz, S.; Stephan, A.; Hahn, S.; Bortesi, L.; Jarczowski, F.; Bettmann, U.; Paschke, A.K.; Tusé, D.; Stahl, C.H.; Giritch, A.; et al. Broad and efficient control of major foodborne pathogenic strains of Escherichia coli by mixtures of plant-produced colicins. Proc. Natl. Acad. Sci. USA 2015, 112, E5454–E5460. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, J.A.; Ageitos, J.M.; Poza, M.; Villa, T.G. Short communication: A comparative analysis of recombinant chymosins. J. Dairy Sci. 2012, 95, 609–613. [Google Scholar] [CrossRef]
- Palmieri, N.; Forleo, M.B.; Salimei, E. Environmental impacts of a dairy cheese chain including whey feeding: An Italian case study. J. Clean. Prod. 2017, 140, 881–889. [Google Scholar] [CrossRef]
- Murray, J.D.; Nancarrowb, C.D.; Marshall, J.T.; Hazeltonn, I.G.; Ward, K.A. Production of transgenic merino sheep by microinjection of ovine metallothionein-ovine growth hormone fusion genes. Reprod. Fertil. Dev. 1989, 1, 147–155. [Google Scholar] [CrossRef]
- Ittner, L.M.; Götz, J. Pronuclear injection for the production of transgenic mice. Nat. Protoc. 2007, 2, 1206–1215. [Google Scholar] [CrossRef] [Green Version]
- Powell, B.C.; Walker, S.K.; Bawden, C.S.; Sivaprasad, A.V.; Rogers, G.E. Transgenic sheep and wool growth: Possibilities and current status. Reprod. Fertil. Dev. 1994, 6, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Brophy, B.; Smolenski, G.; Wheeler, T.; Wells, D.; L’Huillier, P.; Laible, G. Cloned transgenic cattle produce milk with higher levels of β-casein and κ-casein. Nat. Biotechnol. 2003, 21, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, Z.; Yu, T.; Ding, F.; Li, L.; Wang, X.; Fu, M.; Wang, H.; Huang, J.; Li, N.; et al. Large-scale production of recombinant human lactoferrin from high-expression, marker-free transgenic cloned cows. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.A.; Kanwar, R.K.; Kanwar, J.R. Lactoferrin and cancer in different cancer models. Front. Biosci. 2011, 3, 1080–1088. [Google Scholar] [CrossRef] [Green Version]
- Donovan, D.M.; Kerr, D.E.; Wall, R.J. Engineering disease resistant cattle. Transgenic Res. 2005, 14, 563–567. [Google Scholar] [CrossRef]
- Venters, G.A. New variant Creutzfeldt-Jakob disease: The epidemic that never was. Br. Med. J. 2001, 323, 858–861. [Google Scholar] [CrossRef] [Green Version]
- Richt, J.A.; Kasinathan, P.; Hamir, A.N.; Castilla, J.; Sathiyaseelan, T.; Vargas, F.; Sathiyaseelan, J.; Wu, H.; Matsushita, H.; Koster, J.; et al. Production of cattle lacking prion protein. Nat. Biotechnol. 2007, 25, 132–138. [Google Scholar] [CrossRef]
- Maga, E.A.; Shoemaker, C.F.; Rowe, J.D.; BonDurant, R.H.; Anderson, G.B.; Murray, J.D. Production and processing of milk from transgenic goats expressing human lysozyme in the mammary gland. J. Dairy Sci. 2006, 89, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Laible, G.; Wei, J.; Wagner, S. Improving livestock for agriculture—Technological progress from random transgenesis to precision genome editing heralds a new era. Biotechnol. J. 2015, 10, 109–120. [Google Scholar] [CrossRef]
- Ruan, J.; Xu, J.; Chen-Tsai, R.Y.; Li, K. Genome editing in livestock: Are we ready for a revolution in animal breeding industry? Transgenic Res. 2017, 26, 715–726. [Google Scholar] [CrossRef]
- Egelie, K.J.; Graff, G.D.; Strand, S.P.; Johansen, B. The emerging patent landscape of CRISPR-Cas gene editing technology. Nat. Biotechnol. 2016, 34, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Parrott, W. Outlaws, old laws and no laws: The prospects of gene editing for agriculture in United States. Physiol. Plant. 2018, 164, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Cotta, M.A.; Whitehead, T.R.; Rasmussen, M.A. Survival of the recombinant Bacteroides thetaiotaomicron strain BTX in in vitro rumen incubations. J. Appl. Microbiol. 1997, 82, 743–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, G.O.; Badhan, A.; Huang, J.; Beauchemin, K.A.; Yang, W.; Wang, Y.; Tsang, A.; McAllister, T.A. New recombinant fibrolytic enzymes for improved in vitro ruminal fiber degradability of barley straw. J. Anim. Sci. 2018, 96, 3928–3942. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.; Ávila, P.F.; Paim de Andrade, C.C.; Goldbeck, R. Synergic recombinant enzyme association to optimize xylo-oligosaccharides production from agricultural waste. Biocatal. Agric. Biotechnol. 2020, 28, 101747. [Google Scholar] [CrossRef]
- Wu, D.; Wang, S.; Vinitchaikul, P.; Zhu, Y.; Zhou, X.; Gu, Z.; Leng, J.; Gou, X.; Deng, M.; Sun, L.; et al. Directed modification of a ruminal cellulase gene (CMC-1) from a metagenomic library isolated from Yunnan gayal (Bos frontalis). Arch. Microbiol. 2020, 202, 1117–1126. [Google Scholar] [CrossRef]
- Duan, X.; Shen, Z.; Zhang, X.; Wang, Y.; Huang, Y. Production of recombinant beta-amylase of Bacillus aryabhattai. Prep. Biochem. Biotechnol. 2019, 49, 88–94. [Google Scholar] [CrossRef]
- Liu, J.R.; Duan, C.H.; Zhao, X.; Tzen, J.T.C.; Cheng, K.J.; Pai, C.K. Cloning of a rumen fungal xylanase gene and purification of the recombinant enzyme via artificial oil bodies. Appl. Microbiol. Biotechnol. 2008, 79, 225–233. [Google Scholar] [CrossRef]
- Chen, W.; Chen, H.; Xia, Y.; Zhao, J.; Tian, F.; Zhang, H. Production, purification, and characterization of a potential thermostable galactosidase for milk lactose hydrolysis from Bacillus stearothermophilus. J. Dairy Sci. 2008, 91, 1751–1758. [Google Scholar] [CrossRef]
- Robitaille, G.; Tremblay, A.; Moineau, S.; St-Gelais, D.; Vadeboncoeur, C.; Britten, M. Fat-free yogurt made using a galactose-positive exopolysaccharide-producing recombinant strain of Streptococcus thermophilus. J. Dairy Sci. 2009, 92, 477–482. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Cui, Y.; Zhang, L.; Zhang, L.; Liu, H.; Yu, J. Optimization of recombinant Zea mays transglutaminase production and its influence on the functional properties of yogurt. Food Sci. Biotechnol. 2017, 26, 723–730. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pech-Cervantes, A.A.; Irfan, M.; Estrada-Reyes, Z.M.; Ogunade, I.M. Recombinant Technologies to Improve Ruminant Production Systems: The Past, Present and Future. Processes 2020, 8, 1633. https://doi.org/10.3390/pr8121633
Pech-Cervantes AA, Irfan M, Estrada-Reyes ZM, Ogunade IM. Recombinant Technologies to Improve Ruminant Production Systems: The Past, Present and Future. Processes. 2020; 8(12):1633. https://doi.org/10.3390/pr8121633
Chicago/Turabian StylePech-Cervantes, Andres Alfredo, Muhammad Irfan, Zaira Magdalena Estrada-Reyes, and Ibukun Michael Ogunade. 2020. "Recombinant Technologies to Improve Ruminant Production Systems: The Past, Present and Future" Processes 8, no. 12: 1633. https://doi.org/10.3390/pr8121633
APA StylePech-Cervantes, A. A., Irfan, M., Estrada-Reyes, Z. M., & Ogunade, I. M. (2020). Recombinant Technologies to Improve Ruminant Production Systems: The Past, Present and Future. Processes, 8(12), 1633. https://doi.org/10.3390/pr8121633