Novel Insights on the Sustainable Wet Mode Fractionation of Black Soldier Fly Larvae (Hermetia illucens) into Lipids, Proteins and Chitin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solvents, Standards and Reagents
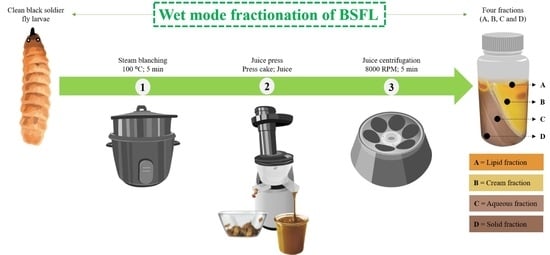
2.2. Wet Mode Fractionation Methodology
2.3. Fatty Acid Profile and Lipid Class Determination
2.4. Protein Quality Parameters and Functional Properties
2.5. Molecular Weight Distribution of Proteins
2.6. Amino Acid Composition and Chitin Quantitation
2.7. Total Phenolics and Trolox Equivalent Antioxidant Capacity (TEAC) Assays
2.8. Statistical Analysis
3. Results and Discussion
3.1. Energy Consumption and Material Distribution
3.2. Proximate Composition of BSFL Juice Fractions
3.3. Fatty Acid Profile and Lipid Class Constituents
3.4. Molecular Weight Distribution of Proteins
3.5. Protein Functional Properties and Quality Parameters
3.6. Amino Acid Distribution in Wet Mode BSFL Fractions
3.7. Antioxidant Potential of Aqueous Fraction BSFL
3.8. Industrial Relevance and Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomberlin, J.K.; van Huis, A. Black soldier fly from pest to “crown jewel” of the insects as feed industry: An historical perspective. J. Insects Food Feed 2020, 6, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Jiang, C.; Zhang, Z.; Lu, W.; Wang, H. Material flow analysis and life cycle assessment of food waste bioconversion by black soldier fly larvae (Hermetia illucens L.). Sci. Total Environ. 2021, 750, 141656. [Google Scholar] [CrossRef]
- Ravi, H.K.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F.; Vian, M.A. Larvae mediated valorization of industrial, agriculture and food wastes: Biorefinery concept through bioconversion, processes, procedures, and products. Processes 2020, 8, 857. [Google Scholar] [CrossRef]
- Jucker, C.; Lupi, D.; Moore, C.D.; Leonardi, M.G.; Savoldelli, S. Nutrient recapture from insect farm waste: Bioconversion with Hermetia illucens (L.) (Diptera: Stratiomyidae). Sustainability 2020, 12, 362. [Google Scholar] [CrossRef] [Green Version]
- European Commission. European Union United States is Europe’ s Main Soya Beans Supplier with Imports Up by 112%; European Union: Maastricht, The Netherlands, 2019; pp. 28–29. [Google Scholar]
- Abd El-Hack, M.E.; Shafi, M.E.; Alghamdi, W.Y.; Abdelnour, S.A.; Shehata, A.M.; Noreldin, A.E.; Ashour, E.A.; Swelum, A.A.; Al-sagan, A.A.; Alkhateeb, M.; et al. Black soldier fly (Hermetia illucens) meal as a promising feed ingredient for poultry: A comprehensive review. Agriculture 2020, 10, 339. [Google Scholar] [CrossRef]
- Bessa, L.W.; Pieterse, E.; Marais, J.; Hoffman, L.C. Why for feed and not for human consumption? The black soldier fly larvae. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2747–2763. [Google Scholar] [CrossRef]
- Gasco, L.; Biasato, I.; Dabbou, S.; Schiavone, A.; Gai, F. Animals fed insect-based diets: State-of-the-art on digestibility, performance and product quality. Animals 2019, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, 1–29. [Google Scholar]
- Ravi, H.K.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F.; Vian, M.A. Effect of devitalization techniques on the lipid, protein, antioxidant, and chitin fractions of black soldier fly (Hermetia illucens) larvae. Eur. Food Res. Technol. 2020, 246, 2549–2568. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Azzollini, D.; van Iwaarden, A.; Lakemond, C.M.M.; Fogliano, V. Mechanical and enzyme assisted fractionation process for a sustainable production of black soldier fly (Hermetia illucens) ingredients. Front. Sustain. Food Syst. 2020, 4, 1–9. [Google Scholar] [CrossRef]
- Smets, R.; Verbinnen, B.; Van De Voorde, I.; Aerts, G.; Claes, J.; Van Der Borght, M. Sequential extraction and characterisation of lipids, proteins, and chitin from black soldier fly (Hermetia illucens) larvae, prepupae, and pupae. Waste Biomass Valorization 2020, 11, 6455–6466. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.P.; Van Den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Ravi, H.K.; Breil, C.; Vian, M.A.; Chemat, F.; Venskutonis, P.R. Biorefining of bilberry (Vaccinium myrtillus L.) pomace using microwave hydrodiffusion and gravity, ultrasound-assisted, and bead-milling extraction. ACS Sustain. Chem. Eng. 2018, 6, 4185–4193. [Google Scholar] [CrossRef]
- Gharby, S.; Ravi, H.K.; Guillaume, D.; Abert Vian, M.; Chemat, F.; Charrouf, Z. 2-methyloxolane as alternative solvent for lipid extraction and its effect on the cactus (Opuntia ficus-indica L.) seed oil fractions. OCL 2020, 27, 27. [Google Scholar] [CrossRef]
- Van Eys, J.E. Manual of Quality Analyses for Soybean Products in the Feed Industry, 2nd ed.; U.S. Soybean Export Council: Singapore, 2012. [Google Scholar]
- Batish, I.; Brits, D.; Valencia, P.; Miyai, C.; Rafeeq, S.; Xu, Y.; Galanopoulos, M.; Sismour, E.; Ovissipour, R. Effects of enzymatic hydrolysis on the functional properties, antioxidant activity and protein structure of black soldier fly (Hermetia illucens) protein. Insects 2020, 11, 876. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Soetemans, L.; Uyttebroek, M.; D’Hondt, E.; Bastiaens, L. Use of organic acids to improve fractionation of the black soldier fly larvae juice into lipid- and protein-enriched fractions. Eur. Food Res. Technol. 2019, 245, 2257–2267. [Google Scholar] [CrossRef] [Green Version]
- Matthäus, B.; Piofczyk, T.; Katz, H.; Pudel, F. Renewable resources from insects: Exploitation, properties, and refining of fat obtained by cold-pressing from Hermetia illucens (Black Soldier Fly) larvae. Eur. J. Lipid Sci. Technol. 2019, 121, 1–11. [Google Scholar] [CrossRef]
- Bertrand, H.; Frédéric, F.; Joachim, C.; Lucien, M.; Christophe, B.; Giorgia, P.; Megido Rudy, C. ω3-enrichment of Hermetia illucens (L. 1758) prepupae from oilseed byproducts. J. Saudi Soc. Agric. Sci. 2021, 20, 155–163. [Google Scholar]
- Xu, X.; Ji, H.; Belghit, I.; Liland, N.S.; Wu, W.; Li, X. Effects of black soldier fly oil rich in n-3 HUFA on growth performance, metabolism and health response of juvenile mirror carp (Cyprinus carpio var. specularis). Aquaculture 2021, 533, 736144. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Sorci, A.; Bonzanini, F.; Lolli, V.; Maistrello, L.; Sforza, S. Influence of the killing method of the black soldier fly on its lipid composition. Food Res. Int. 2018, 116, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Ravi, H.K.; Vian, M.A.; Tao, Y.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F. Alternative solvents for lipid extraction and their effect on protein quality in black soldier fly (Hermetia illucens) larvae. J. Clean. Prod. 2019, 238, 117861. [Google Scholar] [CrossRef]
- Smets, R.; Goos, P.; Claes, J.; Van Der Borght, M. Optimisation of the lipid extraction of fresh black soldier fly larvae (Hermetia illucens) with 2-methyltetrahydrofuran by response surface methodology. Sep. Purif. Technol. 2021, 258, 118040. [Google Scholar] [CrossRef]
- Wang, T.; Shen, Q.; Feng, W.; Wang, C.; Yang, F. Aqueous ethyl acetate as a novel solvent for the degreasing of black soldier fly (Hermetia illucens L.) larvae: Degreasing rate, nutritional value evaluation of the degreased meal, and thermal properties. J. Sci. Food Agric. 2020, 100, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Ishak, S.; Kamari, A.; Yusoff, S.N.M.; Halim, A.L.A. Optimisation of biodiesel production of black soldier fly larvae rearing on restaurant kitchen waste. J. Phys. Conf. Ser. 2018, 1097, 012052. [Google Scholar] [CrossRef] [Green Version]
- Bußler, S.; Rumpold, B.A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Recovery and techno-functionality of flours and proteins from two edible insect species: Meal worm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon 2016, 2, e00218. [Google Scholar] [CrossRef] [PubMed]
- Rabani, V.; Cheatsazan, H.; Davani, S. Proteomics and lipidomics of black soldier fly (Diptera: Stratiomyidae) and blow fly (Diptera: Calliphoridae) larvae. J. Insect Sci. 2019, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Miron, L.T.; Postma, R.P.; Bosch, G.; Eppink, M.H.M. Preliminary evaluation of aqueous protein extraction from black soldier fly larvae (Hermetia illucens L.). Ind. Biotechnol. 2019, 15, 365–369. [Google Scholar] [CrossRef]
- Leni, G.; Tedeschi, T.; Faccini, A.; Pratesi, F.; Folli, C.; Puxeddu, I.; Migliorini, P.; Gianotten, N.; Jacobs, J.; Depraetere, S. Shotgun proteomics, in-silico evaluation and immunoblotting assays for allergenicity assessment of lesser mealworm, black soldier fly and their protein hydrolysates. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Yang, J.; Yao, L.; Qin, F.; Hou, G.; Chen, B.; Jin, L.; Deng, J.; Shen, Y. Characterisation, physicochemical and functional properties of protein isolates from Amygdalus pedunculata Pall seeds. Food Chem. 2020, 311, 125888. [Google Scholar] [CrossRef]
- Purschke, B.; Meinlschmidt, P.; Horn, C.; Rieder, O.; Jäger, H. Improvement of techno-functional properties of edible insect protein from migratory locust by enzymatic hydrolysis. Eur. Food Res. Technol. 2018, 244, 999–1013. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, E.; Karaś, M.; Baraniak, B. Comparison of functional properties of edible insects and protein preparations thereof. LWT Food Sci. Technol. 2018, 91, 168–174. [Google Scholar] [CrossRef]
- Hall, F.G.; Jones, O.G.; O’Haire, M.E.; Liceaga, A.M. Functional properties of tropical banded cricket (Gryllodes sigillatus) protein hydrolysates. Food Chem. 2017, 224, 414–422. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.; Sagis, L.M.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Gravel, A.; Doyen, A. The use of edible insect proteins in food: Challenges and issues related to their functional properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Kim, E.J.; Utterback, P.L.; Parsons, C.M. Comparison of amino acid digestibility coefficients for soybean meal, canola meal, fish meal, and meat and bone meal among 3 different bioassays. Poult. Sci. 2012, 91, 1350–1355. [Google Scholar] [CrossRef]
- Hamre, K.; Kolås, K.; Sandnes, K. Protection of fish feed, made directly from marine raw materials, with natural antioxidants. Food Chem. 2010, 119, 270–278. [Google Scholar] [CrossRef]
- Di Mattia, C.; Battista, N.; Sacchetti, G.; Serafini, M. Antioxidant activities in vitro of water and liposoluble extracts obtained by different species of edible insects and invertebrates. Front. Nutr. 2019, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, R.; Spinelli, R.; Neri, P.; Pini, M.; Barbi, S.; Montorsi, M.; Maistrello, L.; Marseglia, A.; Caligiani, A.; Ferrari, A.M. Life cycle assessment of chemical vs. enzymatic-assisted extraction of proteins from black soldier fly prepupae for the preparation of biomaterials for potential agricultural use. ACS Sustain. Chem. Eng. 2020, 8, 14752–14764. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Mäkinen, O.E.; Rommi, K.; Heiniö, R.L.; Holopainen-Mantila, U.; Hokkanen, S.; Hakala, T.K.; Nordlund, E. Biochemical and sensory characteristics of the cricket and mealworm fractions from supercritical carbon dioxide extraction and air classification. Eur. Food Res. Technol. 2018, 244, 19–29. [Google Scholar] [CrossRef]
- Bolat, B.; Ugur, A.E.; Oztop, M.H.; Alpas, H. Effects of high hydrostatic pressure assisted degreasing on the technological properties of insect powders obtained from Acheta domesticus & Tenebrio molitor. J. Food Eng. 2021, 292, 110359. [Google Scholar]
- Ojha, S.; Bußler, S.; Psarianos, M.; Rossi, G.; Schlüter, O.K. Edible insect processing pathways and implementation of emerging technologies. J. Insects Food Feed 2021, 7, 877–900. [Google Scholar] [CrossRef]
- Alles, M.C.; Smetana, S.; Parniakov, O.; Shorstkii, I.; Toepfl, S.; Aganovic, K.; Heinz, V. Bio-refinery of insects with Pulsed electric field pre-treatment. Innov. Food Sci. Emerg. Technol. 2020, 64, 120403. [Google Scholar] [CrossRef]







| Unit Operations | Energy Consumption (kWh) |
|---|---|
| Boiling 1 L of water | 0.169 |
| Steam blanching 500 g of fresh larvae | 0.07 |
| Juice press—500 g of steam-blanched larvae | 0.008 |
| Homogenization (10 × 1000 RPM) | 0.005 |
| Centrifugation at 8000 RPM for 5 min | 0.069 |
| Unit kWh—Kilowatt-hours. |
| Fatty Acids (%)/Sample ID | BSFLSBFD | LFCtrl | LFHg | LFEt | CFCtrl | CFHg | CFEt | SFCtrl | SFHg | SFEt | JPC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C10 | 1.12 | 1.25 | 1.20 | 1.16 | 1.18 | 1.27 | 1.20 | 1.35 | 1.33 | 1.31 | 1.27 |
| C12 | 44.52 | 47.61 | 46.78 | 46.28 | 46.62 | 47.42 | 44.93 | 48.26 | 47.88 | 49.49 | 49.09 |
| C14 | 3.96 | 3.12 | 3.34 | 3.01 | 2.42 | 2.05 | 3.39 | 0.78 | 1.15 | 1.29 | 2.16 |
| C16 | 14.54 | 13.77 | 14.00 | 14.88 | 15.86 | 14.95 | 15.07 | 16.39 | 14.69 | 17.22 | 15.62 |
| C17 | 0.23 | 0.18 | 0.18 | 0.20 | 0.21 | 0.17 | 0.22 | 0.18 | 0.15 | 0.17 | 0.13 |
| C20 | 0.11 | 0.22 | 0.24 | 0.32 | 0.32 | 0.32 | 0.33 | 1.22 | 0.56 | 0.41 | 0.15 |
| ƩSaturated fatty acids | 64.49 | 66.14 | 65.75 | 65.85 | 66.61 | 66.18 | 65.13 | 68.19 | 65.77 | 69.89 | 68.41 |
| C16:1 | 2.13 | 2.17 | 2.16 | 2.19 | 2.25 | 2.39 | 2.16 | 1.30 | 2.54 | 1.20 | 1.13 |
| C18:1n9 | 11.25 | 9.92 | 10.37 | 10.42 | 10.18 | 10.18 | 11.77 | 8.91 | 9.29 | 8.24 | 9.62 |
| ƩMonounsaturated fatty acids | 13.38 | 12.10 | 12.53 | 12.61 | 12.43 | 12.57 | 13.93 | 10.21 | 11.83 | 9.44 | 10.76 |
| C18:2n6 | 19.81 | 19.72 | 19.86 | 19.75 | 19.80 | 20.12 | 19.28 | 19.57 | 20.63 | 19.12 | 19.39 |
| C18:3n3 | 1.01 | 0.87 | 0.81 | 0.77 | 0.25 | 0.14 | 0.54 | 0.10 | 0.12 | 0.14 | 0.09 |
| ƩPolyunsaturated fatty acids | 20.82 | 20.60 | 20.67 | 20.52 | 20.05 | 20.26 | 19.82 | 19.67 | 20.75 | 19.26 | 19.49 |
| Other fatty acids | 1.30 | 1.17 | 1.05 | 1.02 | 0.91 | 0.99 | 1.12 | 1.92 | 1.65 | 1.41 | 1.34 |
| Sample ID | Crude Protein (%) | PDI (%) | PS (%) | OBC (g Oil/g DM) |
|---|---|---|---|---|
| SFCtrl | 51.75 ± 0.36 | 32.41 ± 1.46 b | 68.25 ± 1.41 b | 2.69 ± 0.15 a |
| SFHg | 50.6 ± 0.48 | 27.44 ± 1.21 c | 63.68 ± 1.31 c | 2.68 ± 0.05 a |
| SFEt | 45.32 ± 0.43 | 76.68 ± 1.56 a | 77.05 ± 1.56 a | 2.8 ± 0.04 a |
| JPC | 59.15 ± 1.16 | 18.51 ± 2.57 | 76.94 ± 2.21 | 5.91 ± 0.05 |
| Amino Acid/Sample | JPC | SFCtrl | AFCtrl | SFEt | AFEt | SBM # | FM # |
|---|---|---|---|---|---|---|---|
| Alanine | 6.15 | 2.80 | 2.25 | 2.22 | 3.10 | 2.04 | 4.03 |
| Arginine * | 2.81 | 2.97 | 1.77 | 2.19 | 2.08 | 3.53 | 3.82 |
| Aspartic acid | 4.65 | 6.51 | 3.55 | 5.22 | 6.39 | 5.52 | 5.79 |
| Cysteine + Cystine | 0.41 | 0.68 | 0.40 | 0.56 | 0.53 | 0.77 | 0.6 |
| Glutamic acid | 5.82 | 6.51 | 7.05 | 5.46 | 8.07 | 8.56 | 8.3 |
| Glycine | 4.50 | 2.55 | 1.78 | 2.15 | 2.66 | 2 | 4.8 |
| Histidine * | 2.04 | 1.88 | 2.33 | 1.62 | 1.92 | 1.26 | 1.45 |
| Isoleucine * | 2.64 | 2.63 | 1.03 | 2.21 | 2.30 | 2.26 | 2.66 |
| Leucine * | 4.59 | 4.22 | 1.45 | 3.45 | 3.53 | 3.78 | 4.61 |
| Lysine * | 3.15 | 4.52 | 1.85 | 3.44 | 4.11 | 3.13 | 4.98 |
| Methionine * | 0.73 | 1.39 | 0.33 | 1.18 | 1.15 | 0.68 | 1.8 |
| Phenylalanine * | 2.10 | 2.94 | 1.04 | 2.59 | 2.29 | 2.4 | 2.43 |
| Proline | 7.44 | 2.42 | 1.29 | 2.10 | 2.22 | 2.21 | 2.7 |
| Serine | 3.26 | 2.18 | 1.14 | 1.74 | 2.13 | 2.06 | 2.1 |
| Threonine * | 2.40 | 2.47 | 1.13 | 1.95 | 2.33 | 1.82 | 2.49 |
| Tryptophan * | 1.14 | 1.09 | 0.57 | 0.93 | 0.79 | 0.7 | 1.95 |
| Tyrosine | 4.88 | 3.54 | 1.59 | 2.83 | 3.04 | 1.78 | 3.14 |
| Valine * | 5.16 | 2.97 | 1.42 | 2.48 | 2.79 | 2.4 | 4.03 |
| TAA | 63.88 | 54.27 | 31.96 | 44.31 | 51.42 | 46.9 | 61.68 |
| Ʃ EAA | 26.76 | 27.08 | 12.91 | 22.04 | 23.29 | 21.96 | 30.22 |
| % EAA/TAA | 41.90 | 49.90 | 40.39 | 49.73 | 45.28 | 46.82 | 48.99 |
| TEAC | FCRC | ABTS | DPPH | FRAP |
|---|---|---|---|---|
| Sample ID/Unit | mg GAE/g DM | mg TE/g DM | ||
| AFCtrl | 32.27 ± 3.25 b | 0.42 ± 0.01 b | 0.16 ± 0.03 a | 0.44 ± 0.03 a |
| AFHg | 34.49 ± 1.56 b | 0.51 ± 0.02 b | 0.12 ± 0.03 ab | 0.45 ± 0.01 a |
| AFEt | 55.95 ± 1.07 a | 0.86 ± 0.09 a | 0.08 ± 0.01 b | 0.23 ± 0.07 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravi, H.K.; Guidou, C.; Costil, J.; Trespeuch, C.; Chemat, F.; Vian, M.A. Novel Insights on the Sustainable Wet Mode Fractionation of Black Soldier Fly Larvae (Hermetia illucens) into Lipids, Proteins and Chitin. Processes 2021, 9, 1888. https://doi.org/10.3390/pr9111888
Ravi HK, Guidou C, Costil J, Trespeuch C, Chemat F, Vian MA. Novel Insights on the Sustainable Wet Mode Fractionation of Black Soldier Fly Larvae (Hermetia illucens) into Lipids, Proteins and Chitin. Processes. 2021; 9(11):1888. https://doi.org/10.3390/pr9111888
Chicago/Turabian StyleRavi, Harish Karthikeyan, Côme Guidou, Jérôme Costil, Christophe Trespeuch, Farid Chemat, and Maryline Abert Vian. 2021. "Novel Insights on the Sustainable Wet Mode Fractionation of Black Soldier Fly Larvae (Hermetia illucens) into Lipids, Proteins and Chitin" Processes 9, no. 11: 1888. https://doi.org/10.3390/pr9111888
APA StyleRavi, H. K., Guidou, C., Costil, J., Trespeuch, C., Chemat, F., & Vian, M. A. (2021). Novel Insights on the Sustainable Wet Mode Fractionation of Black Soldier Fly Larvae (Hermetia illucens) into Lipids, Proteins and Chitin. Processes, 9(11), 1888. https://doi.org/10.3390/pr9111888