Universal Chain-End Coupling Conditions for Brominated Polystyrenes, Polyacrylates, and Polymethacrylates
Abstract
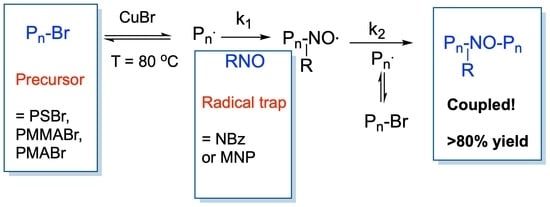
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Typical Procedure for the Synthesis of Monobrominated Polystyrene (PSBr) Using ATRP
2.3. Typical Procedure for RTA-ATRC of PSBr
2.4. Typical Procedure for Thermolysis of PSBr-RTA-ATRC
2.5. Characterization
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jenkins, A.D.; Jones, R.G.; Moad, G. Terminology for reversible-deactivation radical polymerization previously called ‘controlled’ radical or ‘living’ radical polymerization (IUPAC Recommendations 2010). Pure. Appl. Chem. 2009, 82, 483–491. [Google Scholar] [CrossRef]
- Shipp, D.A. Reversible-Deactivation Radical Polymerizations. Polym. Rev. 2011, 51, 99–103. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Stalmach, U.; de Boer, B.; Videlot, C.; van Hutten, P.F.; Hadziioannou, G. Semiconducting Diblock Copolymers Synthesized by Means of Controlled Radical Polymerization Techniques. J. Am. Chem. Soc. 2000, 122, 5464–5472. [Google Scholar] [CrossRef] [Green Version]
- Sarbu, T.; Lin, K.; Ell, J.; Siegwart, D.J.; Spanswick, J.; Matyjaszewski, K. Polystyrene with Designed Molecular Weight Distribution by Atom Transfer Radical Coupling. Macromolecules 2004, 37, 3120–3127. [Google Scholar] [CrossRef]
- Thakur, S.; Tillman, E.S. Efficient metal-free coupling of polystyrene chains using silane radical atom abstraction. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 3488–3493. [Google Scholar] [CrossRef]
- Nottelet, B.; Lacroix-Desmazes, P.; Boutevin, B. Atom transfer radical coupling of polystyrene and poly(methyl acrylate) synthesized by reverse iodine transfer polymerization. Polymer 2007, 48, 50–57. [Google Scholar] [CrossRef]
- Huang, C.F.; Ohta, Y.; Yokoyama, A.; Yokozawa, T. Efficient Low-Temperature Atom Transfer Radical Coupling and Its Application to Synthesis of Well-Defined Symmetrical Polybenzamides. Macromolecules 2011, 44, 4140–4148. [Google Scholar] [CrossRef]
- Voter, A.F.; Tillman, E.S.; Findeis, P.M.; Radzinski, S.C. Synthesis of Macrocyclic Polymers Formed via Intramolecular Radical Trap-Assisted Atom Transfer Radical Coupling. ACS Macro Lett. 2012, 1, 1066–1070. [Google Scholar] [CrossRef]
- Butcher, W.E.; Radzinski, S.C.; Tillman, E.S. Selective formation of diblock copolymers using radical trap-assisted atom transfer radical coupling. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3619–3626. [Google Scholar] [CrossRef]
- Valente, C.J.; Schellenberger, A.M.; Tillman, E.S. Dimerization of Poly(methyl methacrylate) Chains Using Radical Trap-Assisted Atom Transfer Radical Coupling. Macromolecules 2014, 47, 2226–2232. [Google Scholar] [CrossRef]
- Blackburn, S.C.; Myers, K.D.; Tillman, E.S. Macrocyclic poly(methyl acrylate) and macrocyclic poly(methyl acrylate-block-styrene) synthesized by radical trap-assisted atom transfer radical coupling. Macromol. Chem. Phys. 2015, 68, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, S.C. Synthesis of Cyclic Polyacrylate Homopolymers and Diblock Copolymers via RTA-ATRC; Inducing Tacticity by Pi-Pi Stacking; Orthogonality of CuAAC & ATRC. Master’s Thesis, Bucknell University, Lewisburg, PA, USA, 2015. Available online: https://digitalcommons.bucknell.edu/masters_theses/151 (accessed on 14 February 2021).
- Whitfield, R.; Anastasaki, A.; Nikolaou, V.; Jones, G.R.; Engelis, N.G.; Discekici, E.H.; Fleischmann, C.; Willenbacher, J.; Hawker, C.J.; Haddleton, D.M. Universal Conditions for the Controlled Polymerization of Acrylates, Methacrylates, and Styrene via Cu(0)-RDRP. J. Am. Chem. Soc. 2017, 139, 1003–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanda, A.K.; Matyjaszewski, K. Effect of [PMDETA]/[Cu(I)] Ratio, Monomer, Solvent, Counterion, Ligand, and Alkyl Bromide on the Activation Rate Constants in Atom Transfer Radical Polymerization. Macromolecules 2003, 36, 1487–1493. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Braunecker, W.A.; Vacca, A.; Gans, P.; Matyjaszewski, K. Competitive Equilibria in Atom Transfer Radical Polymerization. Macromol. Symp. 2007, 248, 60–70. [Google Scholar] [CrossRef]
- Sarbu, T.; Lin, K.; Spanswick, J.; Gil, R.R.; Siegwart, D.J.; Matyjaszewski, K. Synthesis of Hydroxy-Telechelic Poly(methyl acrylate) and Polystyrene by Atom Transfer Radical Coupling. Macromolecules 2004, 37, 9694–9700. [Google Scholar] [CrossRef]
- Xia, K.; Rubaie, A.; Johnson, B.; Tillman, E.S. ‘Greener’ Coupling of Poly(methyl methacrylate) and Poly(methyl acrylate) Chains using Activators Generated by Electron Transfer and Radical Traps. Macromol. Chem. Phys. 2020, 221, 2000125. [Google Scholar] [CrossRef]
- Arce, M.M.; Pan, C.W.; Thursby, M.M.; Wu, J.P.; Carnicom, E.M.; Tillman, E.S. Influence of Solvent on Radical Trap Assisted Dimerization and Cyclization of Polystyrene Radicals. Macromolecules 2016, 49, 7804–7813. [Google Scholar] [CrossRef]
- Domingues, K.M.; Tillman, E.S. Radical-radical coupling of polystyrene chains using AGET ATRC. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5737–5745. [Google Scholar] [CrossRef]
- Xia, K.; Rubaie, A.J.; Johnson, B.P.; Parker, S.A.; Tillman, E.S. Atom Transfer Coupling Reactions Performed with Benign Reducing Agents and Radical Traps. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2113–2120. [Google Scholar] [CrossRef]
- Carnicom, E.M.; Abruzzese, J.A.; Sidibe, Y.; Myers, K.D.; Tillman, E.S. Effect of Trapping Agent and Polystyrene Chain End Functionality on Radical Trap-Assisted Atom Transfer Radical Coupling. Polymers 2014, 6, 2737–2751. [Google Scholar] [CrossRef] [Green Version]
- Carnicom, E.M.; Coyne, W.E.; Myers, K.D.; Tillman, E.S. One pot, two step sequence converting atom transfer radical polymerization directly to radical trap-assisted atom transfer radical coupling. Polymer 2013, 54, 5560–5567. [Google Scholar] [CrossRef]
- Carnicom, E.M.; Tillman, E.S. Polymerization of Styrene and Cyclization to Macrocyclic Polystyrene in a One-pot, Two-Step Sequence. React. Funct. Polym. 2014, 80, 9–14. [Google Scholar] [CrossRef]
- Wu, J.P.; Pan, C.W.; Heiler, K.E.; Ching, M.E.; Tillman, E.S. Altering the Effectiveness of Radical Traps in Atom Transfer Radical Coupling Reactions of Polymer Chains. Polymer 2017, 127, 66–76. [Google Scholar] [CrossRef]
- Jiang, X.; Vamvakaki, M.; Narain, R. Copper-Catalyzed Bimolecular Coupling of α,ω-Dibromide-Functionalized Poly(γ-caprolactone). Macromolecules 2010, 43, 3228–3232. [Google Scholar] [CrossRef]
- Sasaki, D.; Suzuki, Y.; Hagiwara, T.; Yano, S.; Sawaguchi, T. Synthesis and applications of triblock and multiblock copolymers using telechelic oligopropylene. Polymer 2008, 49, 4094–4100. [Google Scholar] [CrossRef]








| Trial | Radical Trap | T (°C) | Equivalents (Cu0:CuBr: PMDETA:Radical Trap) d | Precursor e Mn f | Precursor Ð g | Coupled Product h Mn | Extent of Coupling (Xc) i |
|---|---|---|---|---|---|---|---|
| 1 | PBN a | 40 | 5:5:10:0.6 | 1570 | 1.08 | 1580 | <0.1 |
| 2 | NBz b | 40 | 5:5:10:0.6 | 1570 | 1.08 | 1680 | 0.13 |
| 3 | NBz | 40 | 2.5:4:8:0.6 | 2060 | 1.08 | 2700 | 0.47 |
| 4 | NBz | 40 | 2.5:4:8:1 | 2900 | 1.1 | 4000 | 0.55 |
| 5 | NBz | 80 | 2.5:4:8:1 | 3520 | 1.06 | 4860 | 0.55 |
| 6 | NBz | 80 | 2.5:4:8:0.6 | 2900 | 1.1 | 4140 | 0.60 |
| 7 | NBz | 80 | 2.5:4:8:1 | 7740 | 1.07 | 11,400 | 0.64 |
| 8 | MNP c | 40 | 2.5:4:8:0.6 | 2050 | 1.17 | 2550 | 0.39 |
| 9 | MNP | 40 | 5:5:10:0.6 | 7670 | 1.19 | 9590 | 0.40 |
| 10 | MNP | 80 | 2.5:4:8:0.6 | 4710 | 1.07 | 7990 | 0.82 |
| 11 | MNP | 80 | 2.5:4:8:1 | 1420 | 1.06 | 2710 | 0.96 |
| Trial | Radical Trap | T (°C) | Equivalents (Cu0:CuBr: PMDETA:Radical Trap) d | Precursor e Mn f | Precursor Ð g | Coupled Product h Mn | Extent of Coupling (Xc) i |
|---|---|---|---|---|---|---|---|
| 12 | PBN a | 40 | 5:5:10:0.6 | 4370 | 1.18 | 5260 | 0.34 |
| 13 | NBz b | 40 | 2.5:4:8:0.6 | 4370 | 1.18 | 6920 | 0.74 |
| 14 | NBz | 40 | 5:5:10:0.6 | 4370 | 1.18 | 7030 | 0.76 |
| 15 | NBz | 80 | 2.5:4:8:0.6 | 2870 | 1.16 | 5360 | 0.93 |
| 16 | NBz | 80 | 2.5:4:8:1 | 2870 | 1.16 | 5070 | 0.87 |
| 17 | MNP c | 80 | 2.5:4:8:1 | 2870 | 1.16 | 3170 | 0.19 |
| Trial | Radical Trap | T (°C) | Equivalents (Cu0:CuBr: PMDETA:Radical Trap) d | Precursor e Mn f | Precursor Ð g | Coupled Product h Mn | Extent of Coupling (Xc) i |
|---|---|---|---|---|---|---|---|
| 18 | PBN a | 40 | 5:5:10:0.6 | 2750 | 1.11 | 3000 | 0.17 |
| 19 | NBz b | 40 | 2.5:4:8:0.6 | 2710 | 1.09 | 4310 | 0.74 |
| 20 | NBz | 40 | 5:5:10:0.6 | 2750 | 1.11 | 4500 | 0.78 |
| 21 | NBz | 80 | 2.5:4:8:0.6 | 3000 | 1.01 | 4460 | 0.65 |
| 22 | NBz | 80 | 2.5:4:8:1 | 2170 | 1.08 | 3400 | 0.72 |
| 23 | MNP c | 40 | 2.5:4:8:0.6 | 2780 | 1.11 | 4730 | 0.82 |
| 24 | MNP | 40 | 5:5:10:0.6 | 2260 | 1.08 | 4080 | 0.90 |
| 25 | MNP | 80 | 2.5:4:8:1 | 3450 | 1.08 | 5950 | 0.84 |
| Polymer | Radical Trap | T (°C) | Equivalents (Cu0:CuBr: PMDETA:Radical Trap) e | Precursor e Mn f | Precursor Ð g | Coupled Product h Mn | Extent of Coupling (Xc) i |
|---|---|---|---|---|---|---|---|
| PMABr c | MNP a | 80 | 2.5:4:8:1 | 1420 | 1.06 | 2710 | 0.96 |
| PMMABr c | NBz b | 80 | 2.5:4:8:1 | 2870 | 1.16 | 5070 | 0.87 |
| PSBr d | MNP | 80 | 2.5:4:8:1 | 3450 | 1.08 | 5950 | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andry, J.J.; Lee, J.J.; Wu, J.; Xia, K.; Tillman, E.S. Universal Chain-End Coupling Conditions for Brominated Polystyrenes, Polyacrylates, and Polymethacrylates. Processes 2021, 9, 1001. https://doi.org/10.3390/pr9061001
Andry JJ, Lee JJ, Wu J, Xia K, Tillman ES. Universal Chain-End Coupling Conditions for Brominated Polystyrenes, Polyacrylates, and Polymethacrylates. Processes. 2021; 9(6):1001. https://doi.org/10.3390/pr9061001
Chicago/Turabian StyleAndry, Joseph J., Jaenic J. Lee, Jessica Wu, Katherine Xia, and Eric S. Tillman. 2021. "Universal Chain-End Coupling Conditions for Brominated Polystyrenes, Polyacrylates, and Polymethacrylates" Processes 9, no. 6: 1001. https://doi.org/10.3390/pr9061001
APA StyleAndry, J. J., Lee, J. J., Wu, J., Xia, K., & Tillman, E. S. (2021). Universal Chain-End Coupling Conditions for Brominated Polystyrenes, Polyacrylates, and Polymethacrylates. Processes, 9(6), 1001. https://doi.org/10.3390/pr9061001