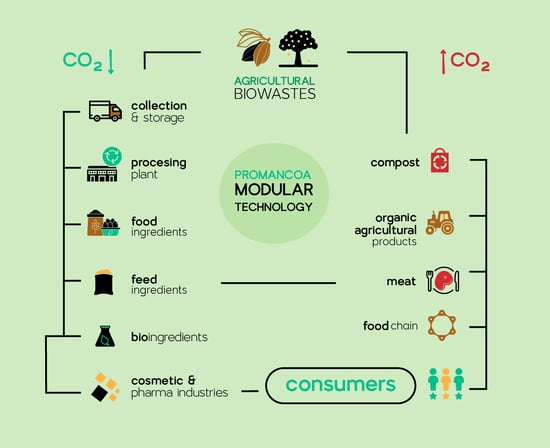
PROMANCOA Modular Technology for the Valorization of Mango (Mangifera indica L.) and Cocoa (Theobroma cacao L.) Agricultural Biowastes
Abstract
:1. Introduction
2. Results
2.1. Collection of Green Raw Material (GRM)
2.1.1. Mango
2.1.2. Cocoa
2.2. GRM Processing
2.2.1. GRM Drying
- Mango: The GRM water content from MSB was reduced from 42.7% to 11.8% with solar drying, and from 11.8% to 5.8% with rotary hot air drying according to results shown in Table 3. The MSB from Baní had a significantly higher water content than the one from Puerto Plata, which corroborated the importance of geographical origin for the GRM yield. However, GRM from both origins had similar water content after the drying operation units, which allowed for the subsequent standardization of the following bioactive green extract (BGE) extraction. MBT, which was allowed to solar dry in the field for a number of weeks, was directly submitted to hot air drying due to its low water content (around 10%). MBT yields were considerably higher compared to MSB, as shown in Table 4. A significant lower yield of MSB was obtained in the Baní farm as compared to the Puerto Plata farm;
- Cocoa: The CPH water content was reduced from an average of 84.5% to 14.6% after solar drying, and from 14.6% to 5.5% after rotary hot air drying, as shown in Table 5. No significant differences were observed between both geographical origins and between different colored pods. The GRM from all origins and varieties had a similar water content for subsequent extraction. The average yield of dried CPH was around 6%.
2.2.2. GRM Milling and Pelletizing
2.2.3. GRM Extraction
- Mango: BGE yields from MSB were higher than MBT for both varieties (Haden and Tommy Atkins). The best yield was obtained from the Haden variety, from the Baní farm, with 12%. The lowest yield was obtained for MBT from the Haden variety, from the Azua farm, however, this GRM had the highest polyphenol content (PPC), and, therefore, the highest mangiferin (MF) content. MF content in BGE polyphenol fraction ranged from 41.6% (MBT Tommy Atkins variety) to 55.4% (MSB Haden variety). The higher MF contents were found for the Haden variety in both MSB and MBT. All the BGE had similar water content. Results of the GRM extraction and quality control are shown in Table 7. Microbiological quality control was performed successfully for all lots (results not shown);
- Cocoa: BGE yields from CBS are shown in Table 8. Since no significant differences were observed in the PPC values for both geographical origin and pod color, the samples were homogenized in order to run the pilot plant batches. Due to the low yields of CPH (below 6%), BGE extraction from this GRM was not performed. Pellets from the CPH will be used for feed formulations and the results will be shown elsewhere. CBS-PPC was around 28 g/100 g and approximately 50% of its content (13.9 g) was a mixture of five different types of OPA.
2.3. Bioactive Ingredients
2.3.1. Mango
2.3.2. Cocoa
3. Discussion
3.1. PROMANCOA Technology Development for Mango Agricultural Biowastes
3.2. PROMANCOA Technology Development for Cocoa Agricultural Biowastes
3.3. PROMANCOA Technology for Other Agricultural Biowastes
4. Materials and Methods
4.1. Module 1: GRM Collection and Storage
4.1.1. Mango Stem Bark (MSB)
- Stem was marked with a chalk at 25 cm below the lowest tree branch and 25 cm above the highest root. Using a compass, the collection was performed first in the stem side looking to the north, and in the south afterwards;
- With the use of a manual circular saw (diameter 5 cm) bark was marked, with not more than 2 cm depth, making a rectangle of around 10 cm width and up to 50 cm height, depending on the tree size;
- The bark was separated without damaging the stem, with specially designed tools, in clockwise direction, first from the north side, and after that from the south side;
- MSB pieces were cleaned manually from dust and residues and collected in sealed polystyrene bags, approximately 50 kg per bag, and identified with a card indicating location, variety, date of collection, and the operator’s name. The bags were stored in the dark (at room temperature), and transported to the processing plant within 7 days of collection.
4.1.2. Mango Branch Trees (MBT)
- Branch trees were cut and left in the field for one week for solar drying;
- Solar dried MBT was milled in the field with a crusher and collected in polystyrene sealed bags, approximately 50 kg per bag, and identified with a card indicating location, variety, date of collection, and operator name. The bags were stored in the dark (at room temperature) and transported to the processing plant within 7 days of collection.
4.1.3. Cocoa Pod Husk (CPH)
- Cocoa plants in the field were selected randomly according to the presence of green-yellowish or red-orange pods. Green-yellowish and red-orange pods were collected separately;
- The outer mass from the pods were collected after a careful longitudinal cut, taking care not to cut the cocoa seeds;
- Crude CPH was collected in plastic tanks (around 200 kg/tank), and seeds were collected in seed containers for subsequent fermentation. CPH water content was around 85%;
- The plastic tanks were transported to the pilot plant within 24 to 48 h after collection for subsequent drying.
4.1.4. Cocoa Bean Shell (CBS)
4.2. Module 2: GRM Processing
4.2.1. GRM Drying
- Mango: Each lot (100 kg) was dried within 72 and 120 h, depending on the initial water content, and divided in two sections, A and B, with manual removal every 24 h. Average solar global radiation was between 5.25 and 5.50 kWh/m2/day [61]. Approximately 2 kg of mango GRM (MSB or MBT) were brought into 304 stainless steel (SS) trays with 60-mesh size steel in the bottom. SS trays were put in the solar drying unit (see Supplementary Information). Hot air drying was subsequently performed in a rotary dryer (Girbau, Barcelona, Spain, Model E660) at 60 °C, with a rotary speed of 80 rpm. The dryer drum was covered with a 60-mesh cloth in order to avoid particle entrance into the dryer motor. Each lot from solar drying was divided in two lots of 50 kg each and dried with hot air for 3 h. MBT had low water content (between 10% and 15%), and therefore solar drying was not necessary;
- Cocoa: Approximately 1.5 kg of CPH was put in each tray of the solar drying unit as described above. Each lot (75 kg) was dried within 96 and 168 h depending on the initial water content, and divided in two dryer sections, A and B, in a similar array as describe for mango. CPH from the solar drying unit was submitted for drying at a rotary hot-air dryer (Girbau, Barcelona, Spain, Model E660) at 60 °C, with a rotary speed of 80 rpm. The dryer drum was covered with a 120-mesh cloth in order to avoid particle entrance into the dryer motor. Each lot from solar drying was divided into two lots of approx. 37 kg each. and dried with hot air for 4 h.
4.2.2. GRM Milling
- Mango: Both MSB and MBT were milled after drying in a hammer mill (Buskirk, Indiana, USA, Model HM1000) equipped with a 20 HP (3 p) motor and screen holes of 10 mm (first step) and 3 mm (second step). Starting GRM sizes were between 5 and 100 mm;
- Cocoa: CBS was milled in the same device in a single step with 3 mm screen holes. Starting CBS sizes were between 2 and 20 mm.
4.2.3. GRM Pelletization
- Pellet Dimension: Diameter and length of cylindrical pellets were measured using a digital Vernier caliper and results were expressed as the mean value ± standard deviation (p > 0.05);
- Pellet Density: 20 individual pellets were weighed in a laboratory balance (Ohaus, NJ, USA, Model FD3H) and its volume calculated according to the Equation (1). Pellet density was calculated according to Equation (2);
- Pellets fines: Pellet fines content was determined using a 1/8-inch (3.18 mm) wire screen sieve (Retsch, Haan, Switzerland, Model AS200);
- Moisture: Pellet moisture (%) was determined with an IR-Balance (Radwag, Puszczykowo, Poland, Model PRM50).
4.3. Module 3: GRM Extraction
4.3.1. Stirred-Tank Extraction
4.3.2. Extract Filtration
4.3.3. Spray Drying
4.4. Module 4: Quality Control
4.4.1. Bioactive Green Extract (BGE)
4.4.2. Bioactive Green Ingredient (BGI)
- Polyphenol Content (PPC): The polyphenol content (PPC) of mango- and cocoa-extracts was determined by a modified Folin-Ciocalteu method using a catechin-equivalent standard [27]. The extracts (1.15 mg) were dissolved in methanol (2 mL) and the solution was diluted ten-fold with distilled water. Folin-Ciocalteu reagent (0.5 mL) was added to the diluted solution, followed by 0.5 mL of sodium carbonate, 100 g/L solution. The absorbance was measured at 700 nm (Thermo Scientific, MA, USA, Genesys 10 spectrophotometer) with a blank sample (water plus reagents) in the reference cell (1 cm-depth quartz). Quantification was performed by plotting the absorbance value in a calibration curve of (+) catechin used as standard phenol;
- Mangiferin: Pure MF was obtained from MSB-BGE and MBT-BGE by recrystallization in a mixture of acetone:water (5:1) through a modified procedure [64]. The purity of the re-crystallized MF was checked by HPLC (Young Lin, Korea) with a quaternary pump (Model YL-9110), autosampler (YL-9150), and a DAD (diode-array detector, YL-9160). The column (RP-18, 5 μm, 250 × 4 mm i.d., Merck, Darmstadt, Germany) was placed in a column oven (YL-9131) at 30 °C. The solvents were degassed (YL-9101) and the injection volume was 20 μL. The mobile phase used was acetic acid (0.1%) in water (A) and acetic acid (0.1%) in methanol (B). The ratio of A:B increased from 9:1 to 1:9 in 35 min at a flow rate of 1 mL/min. The data acquisition and peak integration analysis was performed using Clarity software (Data Apex, Czech Republic);
- OPA-rich Extract: CBS-BGE was defatted with n-hexane in a Soxhlet apparatus, and subsequently extracted with a mixture of ethanol:water (7:3), pH = 6.5 (with acetic acid) in a ratio of 1:5 at room temperature with agitation (30 rpm) for 30 min. The mixture was filtered under vacuum, the filtrate was spray dried and the solid was recrystallized in a mixture of acetone:water (5:1). OPA content (catechin- and epicatechin types) was determined by HPLC-DAD as described above for MF, with the following changes in experimental conditions: in the mobile phase, the formic acid (0.1%) was in water (A) and acetonitrile (B); the ratio of A:B was increased from 1:9 to 9:1 in 35 min; the flow rate was 0.5 mL/min; and the column temperature, 40 °C.
4.4.3. Chemicals and Standards
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bocken, N.M.P.; de Pauw, I.; Bakker, C.A.; van der Grinten, B. Product design and business model strategies for a circular economy. J. Ind. Prod. Eng. 2016, 33, 308–320. [Google Scholar] [CrossRef] [Green Version]
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A Review on Circular Economy: The Expected Transition to a Balanced Interplay of Environmental and Economic Systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Payne, J.; McKeown, P.; Jones, M.D. A circular economy approach to plastic waste. Polym. Degrad. Stab. 2019, 165, 170–181. [Google Scholar] [CrossRef]
- Fischer, A.; Pascucci, S. Institutional incentives in circular economy transition: The case of material use in the Dutch textile industry. J. Clean. Prod. 2017, 155, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M. Management of Fruit Industrial By-Products—A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [Green Version]
- Toop, T.A.; Ward, S.; Oldfield, T.; Hull, M.; Kirby, M.E.; Theodorou, M.K. AgroCycle–developing a circular economy in agriculture. Energy Procedia 2017, 123, 76–80. [Google Scholar] [CrossRef]
- Núñez-Sellés, A.J.; Delgado-Hernández, R.; Garrido-Garrido, G.; García-Rivera, D.; Guevara-García, M.; Pardo-Andreu, G.L. The paradox of natural products as pharmaceuticals: Experimental evidences of a mango stem bark extract. Pharmacol. Res. 2007, 55, 351–358. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E. Antioxidant Properties of Cocoa Beans (Theobroma cacao L.): Influence of Cultivar and Roasting Conditions. Int. J. Food Prop. 2015, 19, 1242–1258. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Sellés, A.J. El Reto de la Terapia Antioxidante; Editorial Académica Española: Madrid, Spain, 2011; 208p, ISBN 978-3846565995. (In Spanish) [Google Scholar]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of polyphenols and their properties. In Polyphenols: Properties, Recovery and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Cambridge, MA, USA, 2018; pp. 3–44. [Google Scholar]
- Hernández-Rodríguez, P.; Pabón Baquero, L.; Rodríguez Larrota, H. Flavonoids: Potential therapeutic agents by their anti-oxidant capacity. In Bioactive Compounds. Health Benefits and Potential Applications; Segura Campos, M.R., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 265–288. [Google Scholar] [CrossRef]
- Pinto, M.; Palmeira, A.; Fernandes, C.; Resende, D.; Sousa, E.; Cidade, H.; Tiritan, M.; Correia-Da-Silva, M.; Cravo, S. From Natural Products to New Synthetic Small Molecules: A Journey through the World of Xanthones. Molecules 2021, 26, 431. [Google Scholar] [CrossRef]
- Vivas, D.B.; Alvarez-Rivera, G.; Ocampo, A.F.G.; Morantes, S.J.; Sánchez-Camargo, A.D.P.; Cifuentes, A.; Parada-Alfonso, F.; Ibánez, E. Supercritical antisolvent fractionation as a tool for enhancing antiproliferative activity of mango seed kernel extracts against colon cancer cells. J. Supercrit. Fluids 2019, 152, 104563. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Valadez-Carmona, L.; Mendiola, J.A.; Ibáñez, E.; Villamiel, M. Structural characterisation of pectin obtained from cacao pod husk. Comparison of conventional and subcritical water extraction. Carbohydr. Polym. 2019, 217, 69–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jangra, A.; Arora, M.K.; Kisku, A.; Sharma, S. The multifaceted role of mangiferin in health and diseases: A review. Adv. Tradit. Med. 2020, 1–25. [Google Scholar] [CrossRef]
- Dominicana On-Line. Productive Sectors: Cocoa. Cocoa Production in Dominican Republic. Available online: http://www.dominicanaonline.org/portal/espanol/cpo_cacao.asp (accessed on 24 April 2020).
- Sangronis, E.; Soto, M.J.; Valero, Y.; Buscema, I. Cascarilla de cacao venezolano como materia prima de infusiones. Arch. Latinoam. Nutr. 2014, 64, 123–130. [Google Scholar]
- Cantele, C.; Rojo-Poveda, O.; Bertolino, M.; Ghirardello, D.; Cardenia, V.; Barbosa-Pereira, L.; Zeppa, G. In Vitro Bioaccessibility and Functional Properties of Phenolic Compounds from Enriched Beverages Based on Cocoa Bean Shell. Foods 2020, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Balentić, J.P.; Ačkar, Đ.; Jokić, S.; Jozinović, A.; Babić, J.; Miličević, B.; Šubarić, D.; Pavlović, N. Cocoa Shell: A By-Product with Great Potential for Wide Application. Molecules 2018, 23, 1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedan, V.; Weber, C.; Do, T.; Fischer, N.; Reich, E.; Rohn, S. HPTLC fingerprint profile analysis of cocoa proanthocyanidins depending on origin and genotype. Food Chem. 2018, 267, 277–287. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.D.L.L.; Borrás-Linares, I.; Lozano-Sánchez, J.; Joven, J.; Fernández-Arroyo, S.; Segura-Carretero, A. Cocoa and Grape Seed Byproducts as a Source of Antioxidant and Anti-Inflammatory Proanthocyanidins. Int. J. Mol. Sci. 2017, 18, 376. [Google Scholar] [CrossRef]
- SOP 04.02.03.21.2019. Procedure for the Collection of Mango Stems Bark in Agricultural Sites; UNEV, Quality Assessment Dept.: Santo Domingo, Dominican Republic, 2019. [Google Scholar]
- SOP 04.02.03.22.2019. Procedure for the Collection of Mango Branch Trees in Agricultural Sites; UNEV, Quality Assessment Dept.: Santo Domingo, Dominican Republic, 2019. [Google Scholar]
- SOP 04.02.03.23.2019. Procedure for the Collection of Coco Pod Husk in Agricultural Sites; UNEV, Quality Assessment Dept.: Santo Domingo, Dominican Republic, 2019. [Google Scholar]
- Sellés, A.J.N.; Rodríguez, M.D.D.; Balseiro, E.R.; Gonzalez, L.N.; Nicolais, V.; Rastrelli, L. Comparison of Major and Trace Element Concentrations in 16 Varieties of Cuban Mango Stem Bark (Mangifera indica L.). J. Agric. Food Chem. 2007, 55, 2176–2181. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, Z.; Fei, B.; Cai, Z. Comparative properties of bamboo and pine pellets. Wood Fiber Sci. 2014, 46, 510–518. [Google Scholar]
- Sellés, A.J.N.; Castro, H.T.V.; Agüero, J.; González-González, J.; Naddeo, F.; De Simone, F.; Rastrelli, L. Isolation and Quantitative Analysis of Phenolic Antioxidants, Free Sugars, and Polyols from Mango (Mangifera indica L.) Stem Bark Aqueous Decoction Used in Cuba as a Nutritional Supplement. J. Agric. Food Chem. 2002, 50, 762–766. [Google Scholar] [CrossRef]
- Luo, F.; Lv, Q.; Zhao, Y.; Hu, G.; Huang, G.; Zhang, J.; Sun, C.; Li, X.; Chen, K. Quantification and Purification of Mangiferin from Chinese Mango (Mangifera indica L.) Cultivars and Its Protective Effect on Human Umbilical Vein Endothelial Cells under H2O2-induced Stress. Int. J. Mol. Sci. 2012, 13, 11260–11274. [Google Scholar] [CrossRef]
- Lerma-Torres, J.M.; Navarro-Ocaña, A.; Calderón-Santoyo, M.; Hernández-Vázquez, L.; Ruiz-Montañez, G.; Ragazzo-Sanchez, J.A. Preparative scale extraction of mangiferin and lupeol from mango (Mangifera indica L.) leaves and bark by different extraction methods. J. Food Sci. Technol. 2019, 56, 4625–4631. [Google Scholar] [CrossRef]
- Selles, A.J.N.; Daglia, M.; Rastrelli, L. The potential role of mangiferin in cancer treatment through its immunomodulatory, anti-angiogenic, apoptopic, and gene regulatory effects. BioFactors 2016, 42, 475–491. [Google Scholar] [CrossRef]
- Imran, M.; Arshad, M.S.; Butt, M.S.; Kwon, J.-H.; Sultan, M.T. Mangiferin: A natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis. 2017, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Núñez Sellés, A.; Villa, D.G.; Rastrelli, L. Mango polyphenols and its protective effects on diseases associated to oxidative stress. Curr. Pharm. Biotechnol. 2015, 16, 272–280. [Google Scholar] [CrossRef]
- Núñez Sellés, A.J.; Espaillat Martinez, V.M.; Nuevas Paz, L. HPLC-DAD and HPLC-ESI-MS Analysis of Polyphenol-rich Extracts from Mango (Mangifera indica L.), Tommy Atkins and Haden Varieties, Cultivated in Dominican Republic. Intl. J. Pharm. Chem. 2020, 6, 77–88. [Google Scholar] [CrossRef]
- Sellés, A.J.N.; Agüero, J.A.; Paz, L.N. GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects. Open Chem. 2021, 19, 27–38. [Google Scholar] [CrossRef]
- Sogi, D.; Siddiq, M.; Greiby, I.; Dolan, K.D. Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 2013, 141, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Dorta, E.; González, M.; Lobo, M.G.; Sanchez-Moreno, C.; de Ancos, B. Screening of phenolic compounds in by-product extracts from mangoes (Mangifera indica L.) by HPLC-ESI-QTOF-MS and multivariate analysis for use as a food ingredient. Food Res. Int. 2014, 57, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Ameer, K.; Shahbaz, H.; Kwon, J.-H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putnik, P.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S.; Jambrak, A.R.; Granato, D.; Montesano, D.; Kovačević, D.B. Novel Food Processing and Extraction Technologies of High-Added Value Compounds from Plant Materials. Foods 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oddoye, E.O.K.; Agyente-Badu, C.K.; Gyedu-Akoto, E. Cocoa and Its By-Products: Identification and Utilization. In Chocolate in Health and Nutrition; Springer Science and Business Media: Berlin, Germany, 2013; pp. 23–37. [Google Scholar]
- Velazquez-Araque, L.; Cárdenas, J. A preliminary study of pelletized Ecuadorian cocoa pod husk for its use as a source of renewable energy. In Proceedings of the 20th World Mult-conference on Systems, Cybernetics and Informatics, Orlando, FL, USA, 5–8 July 2016; pp. 29–33. [Google Scholar]
- Syamsiro, M.; Saptoadi, H.; Tambunan, B. Experimental Investigation on Combustion of Bio-Pellets from Indonesian Cocoa Pod Husk. Asian J. Appl. Sci. 2011, 4, 712–719. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-H.; Tsai, W.-T.; Liu, S.-C.; Lin, Y.-Q. Thermochemical characterization of biochar from cocoa pod husk prepared at low pyrolysis temperature. Biomass-Convers. Biorefinery 2017, 8, 237–243. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Nieto-Figueroa, K.H.; Oomah, B.D. Cocoa (Theobroma cacao L.) pod husk: Renewable source of bioactive compounds. Trends Food Sci. Technol. 2018, 81, 172–184. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Z.; Maximova, S.N.; Payne, M.J.; Guiltinan, M.J. Proanthocyanidin synthesis in Theobroma cacao: Genes encoding anthocyanidin synthase, anthocyanidin reductase, and leucoanthocyanidin reductase. BMC Plant Biol. 2013, 13, 202. [Google Scholar] [CrossRef] [Green Version]
- Valadez-Carmona, L.; Ortiz-Moreno, A.; Ceballos, G.; Mendiola, J.A.; Ibáñez, E. Valorization of cacao pod husk through supercritical fluid extraction of phenolic compounds. J. Supercrit. Fluids 2018, 131, 99–105. [Google Scholar] [CrossRef]
- Valadez-Carmona, L.; Plazola-Jacinto, C.P.; Hernández-Ortega, M.; Hernández-Navarro, M.D.; Villarreal, F.; Necoechea-Mondragón, H.; Ortiz-Moreno, A.; Ceballos, G. Effects of microwaves, hot air and freeze-drying on the phenolic compounds, antioxidant capacity, enzyme activity and microstructure of cacao pod husks (Theobroma cacao L.). Innov. Food Sci. Emerg. Technol. 2017, 41, 378–386. [Google Scholar] [CrossRef]
- Alves, T.V.G.; da Costa, R.S.; Aliakbarian, B.; Casazza, A.A.; Perego, P.; Arruda, M.S.P.; Júnior, J.O.C.S.; Converti, A.; Costa, R.M.R. Bioactive compounds and antioxidant potential for polyphenol-rich cocoa extract obtained by agroindustrial residue. Nat. Prod. Res. 2017, 33, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Cádiz-Gurrea, M.D.L.L.; Lozano-Sanchez, J.; Contreras, M.D.M.; Legeai-Mallet, L.; Fernández-Arroyo, S.; Carretero, A.S. Isolation, comprehensive characterization and antioxidant activities of Theobroma cacao extract. J. Funct. Foods 2014, 10, 485–498. [Google Scholar] [CrossRef]
- Lu, F.; Garcia, J.R.; Van Damme, I.; Westwood, N.J.; Shaw, L.; Robinson, J.S.; Warren, G.; Chatzifragkou, A.; Mason, S.M.; Gomez, L.; et al. Valorisation strategies for cocoa pod husk and its fractions. Curr. Opin. Green Sustain. Chem. 2018, 14, 80–88. [Google Scholar] [CrossRef]
- Hernández-Hernández, C.; Viera-Alcaide, I.; Sillero, A.M.M.; Fernandez-Bolaños, J.; Rodríguez-Gutiérrez, G. Bioactive compounds in Mexican genotypes of cocoa cotyledon and husk. Food Chem. 2018, 240, 831–839. [Google Scholar] [CrossRef] [Green Version]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Zeppa, G.; Stévigny, C. Cocoa Bean Shell—A By-Product with Nutritional Properties and Biofunctional Potential. Nutrients 2020, 12, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzutti, S.; Rodrigues, L.G.G.; Mezzomo, N.; Venturi, V.; Ferreira, S.R.S. Integrated green-based processes using supercritical CO2 and pressurized ethanol applied to recover antioxidant compouds from cocoa (Theobroma cacao) bean hulls. J. Supercrit. Fluids 2018, 135, 52–59. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed Electric Field Assisted Extraction of Bioactive Compounds from Cocoa Bean Shell and Coffee Silverskin. Food Bioprocess Technol. 2018, 11, 818–835. [Google Scholar] [CrossRef]
- Yusof, A.H.M.; Gani, S.S.A.; Zaidan, U.H.; Halmi, M.I.E.; Zainudin, B.H. Optimization of an Ultrasound-Assisted Extraction Condition for Flavonoid Compounds from Cocoa Shells (Theobroma cacao) Using Response Surface Methodology. Molecules 2019, 24, 711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun-Waterhouse, D.; Waterhouse, G.I.; You, L.; Zhang, J.; Liu, Y.; Ma, L.; Dong, Y. Transforming insect biomass into consumer wellness foods: A review. Food Res. Internat. 2016, 89, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Padam, B.S.; Tin, H.S.; Chye, F.Y.; Abdullah, M.I. Banana by-products: An under-utilized renewable food biomass with great potential. J. Food Sci. Technol. 2012, 51, 3527–3545. [Google Scholar] [CrossRef] [Green Version]
- Savadori, L.; Savio, S.; Nicotra, E.; Rumiati, R.; Finucane, M.; Slovic, P. Expert and public perception of risk from biotechnology. Risk Anal. Int. J. 2004, 24, 1289–1299. [Google Scholar] [CrossRef]
- Siegrist, M. The Influence of Trust and Perceptions of Risks and Benefits on the Acceptance of Gene Technology. Risk Anal. 2000, 20, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Curtis, K.R.; McCluskey, J.J.; Wahl, T.I. Consumer Acceptance of Genetically Modified Food Products in the Developing World. Agro BioForum 2004, 7, 70–75. [Google Scholar]
- Silano, V.; Coppens, P.; Larrañaga-Guetaria, A.; Minghetti, P.; Roth-Ehrang, R. Regulations applicable to plant food supplements and related products in the European Union. Food Funct. 2011, 2, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cne.gob.do/wp-content/uploads/2015/11/potencial-solar.pdf (accessed on 13 April 2020).
- Available online: https://www.pelletheat.org/assets/docs/2018/2018_PFI_Standard%20Specification.pdf (accessed on 23 February 2020).
- Feldsine, P.; Abeyta, C.; Andrews, W.H. AOAC INTERNATIONAL Methods Committee Guidelines for Validation of Qualitative and Quantitative Food Microbiological Official Methods of Analysis. J. AOAC Int. 2002, 85, 1187–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vo, T.H.T.; Nguyen, T.D.; Nguyen, Q.H.; Ushakova, N.A. Extraction of Mangiferin from the Leaves of the Mango Tree Mangifera indica and Evaluation of its Biological Activity in Terms of Blockade of α-glucosidase. Pharm. Chem. J. 2017, 51, 806–810. [Google Scholar] [CrossRef]




| Farm | Location | Varieties | No. Plants | Potential of GRM (ton) | |
|---|---|---|---|---|---|
| MSB | MBT | ||||
| Mangos Ecológicos | Matanzas, Bani | Haden | 360 | 4 | - |
| T. Atkins | 810 | - | 20 | ||
| Finca Espaillat | C. Altamira, P. Plata | T. Atkins | 120 | 2 | - |
| Finca Rodriguez | Azua | Haden | 60 | - | 2 |
| Farm | Location | Varieties | No. Plants | Potential of GRM (kg) | |
|---|---|---|---|---|---|
| CPH | CBS | ||||
| El Maizal | Villa Altagracia | Hybrid-Green | 82 | 1640 | 360 |
| Hybrid-Red | 16 | 320 | 80 | ||
| El Ramonal | San Francisco de Macorís | Hybrid-Green | 112 | 2240 | 560 |
| Hybrid-Red | 32 | 640 | 160 | ||
| Farm | Batch | MSB (kg) | Final Water Content (%) | Yield (%) | |||
|---|---|---|---|---|---|---|---|
| Field | Solar | Hot Air | Solar | Hot Air | |||
| P. Plata | SBTA01 | 362 | 211.5 | 200.1 | 5.8 | 58.4 | 55.3 |
| SBTA02 | 396 | 314.2 | 298.5 | 4.9 | 79.3 | 75.3 | |
| SBTA03 | 311 | 208.0 | 198.0 | 5.4 | 66.9 | 63.7 | |
| Mean value± SD | 5.4 ± 0.4 | 68.2 ± 5.5 | 64.8 ± 6.9 | ||||
| Baní | SBH01 | 532 | 236.5 | 222.2 | 6.2 | 44.4 | 41.8 |
| SBH02 | 686 | 424.0 | 390.0 | 5.3 | 61.8 | 56.9 | |
| SBH03 | 885 | 561.3 | 354.4 | 5.5 | 63.4 | 40.0 | |
| Mean value± SD | 5.7 ± 0.5 | 56.5 ± 7.1 | 46.2 ± 8.8 | ||||
| Farm | Batch | MBT (kg) | Water Content (%) | Yield (%) | |||
|---|---|---|---|---|---|---|---|
| Field | Solar | Hot Air | Solar | Hot Air | |||
| Azua | BTH01 | 288 | - | 270.1 | 4.9 | - | 93.8 |
| BTH02 | 365 | - | 340.0 | 5.4 | - | 93.1 | |
| BTH03 | 430 | - | 400.6 | 5.8 | - | 93.2 | |
| Mean value± SD | 5.5 ± 0.4 | 93.4 ± 0.4 | |||||
| Baní | BTTA01 | 445 | - | 429.5 | 7.8 | - | 96.5 |
| BTTA02 | 670 | - | 650.0 | 7.5 | - | 97.1 | |
| BTTA03 | 895 | - | 822.3 | 5.6 | - | 91.8 | |
| Mean value± SD | 6.9 ± 1.0 | - | 95.1 ± 2.8 | ||||
| Farm | Batch | CPH Weight (kg) | Water Content (%) | Yield (%) | |||
|---|---|---|---|---|---|---|---|
| Field | Solar | Hot Air | Solar | Hot Air | |||
| El Maizal | GreenM1 | 400 | 64.4 | 22.5 | 4.8 | 16.1 | 5.6 |
| GreenM2 | 200 | 26.8 | 11.5 | 7.2 | 13.4 | 5.8 | |
| GreenM3 | 200 | 31.9 | 12.0 | 6.5 | 15.6 | 6.0 | |
| Mean value± SD | 6.2 ± 1.0 | 15.2 ± 0.8 | 5.8 ± 0.2 | ||||
| RedM1 | 200 | 27.7 | 10.1 | 5.5 | 13.9 | 5.1 | |
| RedM2 | 200 | 25.2 | 11.0 | 6.0 | 12.6 | 5.5 | |
| RedM3 | 200 | 30.0 | 12.5 | 7.1 | 15.0 | 6.3 | |
| Mean value± SD | 6.3 ± 0.6 | 13.8 ± 1.1 | 5.6 ± 0.7 | ||||
| El Ramonal | GreenR1 | 400 | 67.5 | 19.6 | 5.2 | 16.9 | 4.9 |
| GreenR2 | 400 | 59.8 | 18.0 | 5.0 | 15.0 | 4.5 | |
| GreenR3 | 200 | 32.1 | 12.7 | 4.6 | 16.1 | 6.4 | |
| Mean value± SD | 4.9 ± 0.3 | 16.0 ± 0.2 | 5.8 ± 0.7 | ||||
| RedR1 | 200 | 31.0 | 11.9 | 5.5 | 15.5 | 6.0 | |
| RedR2 | 200 | 28.6 | 12.0 | 6.2 | 14.3 | 6.0 | |
| RedR3 | 200 | 30.9 | 13.0 | 6.8 | 15.5 | 6.5 | |
| Mean value± SD | 6.1 ± 0.7 | 15.1 ± 0.5 | 6.2 ± 0.3 | ||||
| Parameter | MSB | MBT | CPH | CBS |
|---|---|---|---|---|
| Diameter (mm) | 8.2 ± 0.2 | 8.1 ± 0.2 | 8.1 ± 0.3 | 8.2 ± 0.3 |
| Length (mm) | 15.2 ± 0.4 | 15.2 ± 0.4 | 15.4 ± 0.5 | 15.2 ± 0.2 |
| Broken Pellets (%) | 11 ± 3 | 12 ± 3 | 14 ± 2 | 13 ± 3 |
| Density (kg/m3) | 650 ± 15 | 640 ± 17 | 652 ± 20 | 645 ± 18 |
| Fines (%) | 4 ± 1 | 4 ± 1 | 3 ± 1 | 6 ± 1 |
| Retained in sieve 1/8´´ | 98 ± 4 | 98 ± 4 | 98 ± 3 | 96 ± 4 |
| Moisture (%) | 4.8 ± 0.5 | 5.0 ± 0.4 | 5.2 ± 0.4 | 5.0 ± 0.5 |
| Farm | Batch | MSB/MBT | Quality Control | ||||
|---|---|---|---|---|---|---|---|
| GRM (kg) | BGE (kg) | Yield (%) | Water (%) | PPC (g/100 g) | MF (g/100 g) | ||
| P. Plata | ESBTA01 | 32 | 3.1 | 9.7 | 5.2 | 15.6 | 12.5 |
| ESBTA02 | 32 | 3.2 | 10.0 | 4.8 | 14.4 | 12.2 | |
| ESBTA03 | 32 | 3.4 | 10.6 | 3.9 | 21.2 | 18.0 | |
| ESBTA04 | 32 | 3.2 | 10.0 | 5.0 | 15.1 | 11.3 | |
| ESBTA05 | 32 | 3.0 | 9.4 | 4.5 | 16.5 | 12.7 | |
| Mean value± SD | 9.9 ± 0.7 | 4.7 ± 0.8 | 16.2 ± 1.5 | 13.3 ± 2.0 | |||
| Baní | ESBH01 | 32 | 3.8 | 11.9 | 6.0 | 16.4 | 13.9 |
| ESBH01 | 32 | 3.6 | 11.3 | 4.5 | 17.8 | 14.4 | |
| ESBH01 | 32 | 4.0 | 12.5 | 5.5 | 15.0 | 11.3 | |
| ESBH01 | 32 | 3.8 | 11.9 | 5.0 | 14.9 | 10.7 | |
| ESBH01 | 32 | 4.0 | 12.5 | 4.8 | 15.1 | 12.0 | |
| Mean value± SD | 12.0 ± 0.6 a | 5.2 ± 0.8 | 15.8 ± 1.5 | 12.5 ± 1.7 | |||
| EBTTA01 | 32 | 3.3 | 10.2 | 4.0 | 14.0 | 9.8 | |
| EBTTA02 | 32 | 3.0 | 9.4 | 4.4 | 14.2 | 10.6 | |
| EBTTA03 | 32 | 3.1 | 9.7 | 5.2 | 13.4 | 8.1 | |
| EBTTA04 | 32 | 3.2 | 10.0 | 4.2 | 13.8 | 9.2 | |
| EBTTA05 | 32 | 3.0 | 9.4 | 4.0 | 14.2 | 9.2 | |
| Mean value± SD | 9.7 ± 0.5 b | 4.4 ± 0.8 | 13.9 ± 0.5 b | 9.4 ± 1.2 abd | |||
| Azua | EBTH01 | 32 | 2.3 | 7.2 | 5.1 | 15.5 | 13.1 |
| EBTH02 | 32 | 2.5 | 7.8 | 4.8 | 16.2 | 13.0 | |
| EBTH03 | 32 | 2.3 | 7.2 | 4.2 | 16.8 | 12.9 | |
| EBTH04 | 32 | 2.6 | 8.1 | 5.5 | 16.0 | 12.8 | |
| EBTH05 | 32 | 2.8 | 8.8 | 5.0 | 17.4 | 15.7 | |
| Mean value± SD | 7.8 ± 1.0 abc | 4.9 ± 0.7 | 16.2 ± 1.2 c | 13.5 ± 0.8 c | |||
| Batch | CBS | Quality Control | ||||
|---|---|---|---|---|---|---|
| GRM (kg) | BGE (kg) | Yield (%) | Water (%) | PPC (g/100 g) | (PAE g/100 g) | |
| 01 | 32 | 6.4 | 20.0 | 5.0 | 28.2 | 14.1 |
| 02 | 32 | 6.2 | 19.4 | 4.8 | 27.9 | 13.7 |
| 03 | 32 | 6.6 | 20.6 | 5.1 | 28.3 | 14.0 |
| 04 | 32 | 6.0 | 18.8 | 5.2 | 28.0 | 13.9 |
| 05 | 32 | 6.1 | 19.1 | 4.9 | 28.1 | 14.0 |
| Mean value ± SD | 19.5 ± 0.8 | 5.0 ± 0.2 | 28.1 ± 0.2 | 13.9 ± 0.2 | ||
| Component | RT (min) | MSB | MBT | ||||
|---|---|---|---|---|---|---|---|
| Peak Area | % | g/100 g | Peak Area | % | g/100 g | ||
| Gallic acid | 4.5 ± 0.5 | 2343.55 | 1.3 | 0.2 ± 0.1 | 1451.80 | 0.7 | 0.1 ± 0.1 |
| Unknown | 5.8 ± 0.8 | 1180.88 | 0.6 | 0.1 ± 0.1 | 1543.28 | 0.8 | 0.1 ± 0.1 |
| Methyl gallate | 7.2 ± 1.5 | 3454.01 | 1.8 | 0.3 ± 0.1 | 3201.06 | 1.6 | 0.2 ± 0.1 |
| Propyl gallate | 8.4 ± 1.0 | - | - | - | 3255.45 | 1.7 | 0.2 ± 0.1 |
| (+) Catechin | 11.8 ± 1.2 | 9333.03 | 5.0 | 0.8 ± 0.5 | 14,517.77 | 7.4 | 1.0 ± 0.7 |
| (−) Epicatechin | 13.5 ± 1.5 | 11,666.30 | 6.3 | 1.0 ± 0.4 | 8710.67 | 4.4 | 0.6 ± 0.6 |
| Mangiferin | 14.6 ± 0.8 | 141,745.50 | 76.2 | 12.0 ± 0.5 | 152,436.70 | 77.5 | 10.5 ± 0.7 |
| Isomangiferin | 15.0 ± 0.6 | 12,832.93 | 6.9 | 1.1 ± 0.3 | 10,162.44 | 5.2 | 0.7 ± 0.2 |
| Quercetin | 19.6 ± 1.0 | 3499.89 | 1.9 | 0.3 ± 0.2 | 1496.00 | 0.8 | 0.1 ± 0.1 |
| Total | 16.2 ± 1.2 | 13.5 ± 0.8 | |||||
| Component | RT (min) | CBS Extract | ||
|---|---|---|---|---|
| Peak Area | % | g/100 g | ||
| Single polyphenols (total) | 50.6 | 14.2 ± 0.2 | ||
| (+) Catechin | 9.8 ± 1.0 | 16,781.76 | 7.5 | 2.1 ± 0.5 |
| (−) Epicatechin | 11.4 ± 1.2 | 88,703.65 | 39.5 | 11.1 ± 0.4 |
| Quercetin | 20.9 ± 1.5 | 7991.32 | 3.6 | 1.0 ± 0.5 |
| Oligomeric Proanthocyanidins (total) | 49.4 | 13.9 ± 0.2 | ||
| -Dimer | 31.4 ± 2.2 | 16,822.41 | 7.5 | 2.1 ± 0.1 |
| -Trimer | 33.8 ± 2.0 | 21,576.55 | 9.6 | 2.7 ± 0.2 |
| -Tetramer | 36.1 ± 1.8 | 23,973.95 | 10.6 | 3.0 ± 0.3 |
| -Pentamer | 38.2 ± 1.5 | 27,170.48 | 12.1 | 3.4 ± 0.3 |
| -Hexamer | 40.0 ± 3.0 | 21,567.98 | 9.6 | 2.7 ± 0.2 |
| Total | 28.1 ± 0.2 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-Sellés, A.J.; Abril-González, A.J.; Ramil-Mesa, M. PROMANCOA Modular Technology for the Valorization of Mango (Mangifera indica L.) and Cocoa (Theobroma cacao L.) Agricultural Biowastes. Processes 2021, 9, 1312. https://doi.org/10.3390/pr9081312
Núñez-Sellés AJ, Abril-González AJ, Ramil-Mesa M. PROMANCOA Modular Technology for the Valorization of Mango (Mangifera indica L.) and Cocoa (Theobroma cacao L.) Agricultural Biowastes. Processes. 2021; 9(8):1312. https://doi.org/10.3390/pr9081312
Chicago/Turabian StyleNúñez-Sellés, Alberto J., Alejandro J. Abril-González, and Marlen Ramil-Mesa. 2021. "PROMANCOA Modular Technology for the Valorization of Mango (Mangifera indica L.) and Cocoa (Theobroma cacao L.) Agricultural Biowastes" Processes 9, no. 8: 1312. https://doi.org/10.3390/pr9081312
APA StyleNúñez-Sellés, A. J., Abril-González, A. J., & Ramil-Mesa, M. (2021). PROMANCOA Modular Technology for the Valorization of Mango (Mangifera indica L.) and Cocoa (Theobroma cacao L.) Agricultural Biowastes. Processes, 9(8), 1312. https://doi.org/10.3390/pr9081312