An Overview of Herbal Nutraceuticals, Their Extraction, Formulation, Therapeutic Effects and Potential Toxicity
Abstract
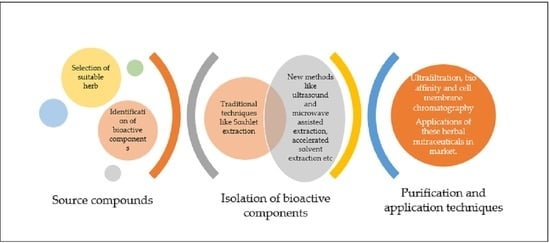
:1. Introduction
Strategy of Searching Articles
2. Sources of Bioactive Compounds (Herbs)
2.1. Aloe Vera
2.2. Angelica
2.3. Anise
2.4. Aralia
2.5. Bay
2.6. Bayberry
2.7. Bee Balm
2.8. Burnet
2.9. Calamint
2.10. Caraway
2.11. Chamomile
2.12. Dill
2.13. Zedoary
2.14. Yarrow
2.15. Wormwood
2.16. Valerian
2.17. Turmeric
2.18. Tulsi (Holy Basil)
2.19. Thyme
2.20. Saffron
2.21. Sage
2.22. Savory
2.23. Rosemary
2.24. Parsley
2.25. Oregano
2.26. Nasturtium
2.27. Mint
2.28. Milk Thistle
2.29. Laurel
2.30. Lemon Grass
2.31. Hyssop
2.32. Gingko
2.33. Ginger
2.34. Garlic
2.35. Fennel
3. Extraction of Bioactive Compounds
3.1. Soxhlet Extraction
3.2. Ultrasound-Assisted Extraction (UAE)
3.3. Microwave-Assisted Extraction (MAE)
3.4. Supercritical Fluid Extraction (SFE)
3.5. Accelerated Solvent Extraction (ASE)
3.6. Hydro-Distillation Extraction
3.7. Ultra High-Pressure Extraction (UPE)
3.8. Enzyme-Assisted Extraction (EAE)
3.9. Pulse Electric Field Extraction (PEF)
4. Purification and Characterization of Bioactive Compounds
4.1. Ultrafiltration
4.2. Bioaffinity Chromatography (BAC)
4.3. Cell Membrane Chromatography (CMC)
4.4. Ligand Fishing
5. Herbal Nutraceuticals in Market
6. Functional Properties of the Nutraceuticals
6.1. Diabetes
6.2. Obesity
6.3. Immunomodulation
6.4. Dementia
6.5. Hypertension
6.6. Antimicrobial Activity
6.7. Hypercholesterolemia
7. Negative Impact of Herbal Nutraceuticals
7.1. Hepatotoxicity
7.2. Pulmonary Toxicity
7.3. Carcinogenicity
7.4. Nephrotoxicity
7.5. Cardiac Toxicity
8. Safety, Quality and Regulatory Aspects of Herbal Nutraceuticals
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ivanišová, E.; Kačániová, M.; Savitskaya, T.D.A.; Grinshpan, D. Medicinal Herbs: Important Source of Bioactive Compounds for Food Industry. In Herbs and Spices—New Processing Technologies; IntechOpen: London, UK, 2021. [Google Scholar]
- Priya, S.; Satheeshkumar, P.K. Natural Products from Plants. In Functional and Preservative Properties of Phytochemicals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 145–163. [Google Scholar]
- Majumder, R.; Das, C.K.; Mandal, M. Lead Bioactive Compounds of Aloe Vera as Potential Anticancer Agent. Pharmacol. Res. 2019, 148, 104416. [Google Scholar] [CrossRef]
- Simpson, M. Plant Systematics, 3rd ed.; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128126288. [Google Scholar]
- Añibarro-Ortega, M.; Pinela, J.; Barros, L.; Ćirić, A.; Silva, S.P.; Coelho, E.; Mocan, A.; Calhelha, R.C.; Soković, M.; Coimbra, M.A.; et al. Compositional Features and Bioactive Properties of Aloe Vera Leaf (Fillet, Mucilage, and Rind) and Flower. Antioxidants 2019, 8, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özenver, N.; Saeed, M.; Demirezer, L.Ö.; Efferth, T. Aloe-Emodin as Drug Candidate for Cancer Therapy. Oncotarget 2018, 9, 17770–17796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huseini, H.; Kianbakht, S.; Hajiaghaee, R.; Dabaghian, F. Anti-Hyperglycemic and Anti-Hypercholesterolemic Effects of Aloe Vera Leaf Gel in Hyperlipidemic Type 2 Diabetic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Planta Med. 2012, 78, 311–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Jia, Y.; Lu, F. Angelica Stem: A Potential Low-Cost Source of Bioactive Phthalides and Phytosterols. Molecules 2018, 23, 3065. [Google Scholar] [CrossRef] [Green Version]
- Bettaieb Rebey, I.; Aidi Wannes, W.; Ben Kaab, S.; Bourgou, S.; Tounsi, M.S.; Ksouri, R.; Fauconnier, M.L. Bioactive Compounds and Antioxidant Activity of Pimpinella Anisum L. Accessions at Different Ripening Stages. Sci. Hortic. 2019, 246, 453–461. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Anise (Pimpinella Anisum L.), a Dominant Spice and Traditional Medicinal Herb for Both Food and Medicinal Purposes. Cogent Biol. 2019, 5, 1673688. [Google Scholar] [CrossRef]
- Ju, B.; Chen, B.; Zhang, X.; Han, C.; Jiang, A. Purification and Characterization of Bioactive Compounds from Styela Clava. J. Chem. 2014, 2014, 525141. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Huang, H.; Xu, C.; Li, X.; Chen, K. Biological Activities of Extracts from Chinese Bayberry (Myrica Rubra Sieb. et Zucc.): A Review. Plant Foods Hum. Nutr. 2013, 68, 97–106. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Zhang, Q.; Fan, F.; Xu, C.; Sun, C.; Li, X.; Chen, K. Phytochemical Characterization of Chinese Bayberry (Myrica Rubra Sieb. et Zucc.) of 17 Cultivars and Their Antioxidant Properties. Int. J. Mol. Sci. 2015, 16, 12467–12481. [Google Scholar] [CrossRef] [Green Version]
- Pelc, M.; Przybyszewska, E.; Przybył, J.L.; Capecka, E.; Bączek, K.; Węglarz, Z. Chemical variability of great burnet (Sanguisorba officinalis L.) growing wild in poland. Acta Hortic. 2011, 925, 97–101. [Google Scholar] [CrossRef]
- Debbabi, H.; El Mokni, R.; Chaieb, I.; Nardoni, S.; Maggi, F.; Caprioli, G.; Hammami, S. Chemical Composition, Antifungal and Insecticidal Activities of the Essential Oils from Tunisian Clinopodium Nepeta Subsp. Nepeta and Clinopodium Nepeta Subsp. Glandulosum. Molecules 2020, 25, 2137. [Google Scholar] [CrossRef]
- Johri, R. Cuminum Cyminum and Carum Carvi: An Update. Pharmacogn. Rev. 2011, 5, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degner, S.C.; Papoutsis, A.J.; Romagnolo, D.F. Health Benefits of Traditional Culinary and Medicinal Mediterranean Plants. In Complementary and Alternative Therapies and the Aging Population; Elsevier: Amsterdam, The Netherlands, 2009; pp. 541–562. [Google Scholar]
- Nour, V.; Trandafir, I.; Cosmulescu, S. Bioactive Compounds, Antioxidant Activity and Nutritional Quality of Different Culinary Aromatic Herbs. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Hadisaputri, Y.E.; Miyazaki, T.; Suzuki, S.; Kubo, N.; Zuhrotun, A.; Yokobori, T.; Abdulah, R.; Yazawa, S.; Kuwano, H. Molecular Characterization of Antitumor Effects of the Rhizome Extract from Curcuma Zedoaria on Human Esophageal Carcinoma Cells. Int. J. Oncol. 2015, 47, 2255–2263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Msaada, K.; Salem, N.; Bachrouch, O.; Bousselmi, S.; Tammar, S.; Alfaify, A.; Al Sane, K.; Ben Ammar, W.; Azeiz, S.; Haj Brahim, A.; et al. Chemical Composition and Antioxidant and Antimicrobial Activities of Wormwood (Artemisia Absinthium L.) Essential Oils and Phenolics. J. Chem. 2015, 2015, 804658. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Pei, S.; He, N.; Lai, Q.; Zhuang, M.; Bian, Z.; Lin, C. A Narrative Review of Botanical Characteristics, Phytochemistry and Pharmacology of Valeriana Jatamansi Jones. Longhua Chinese Med. 2021, 4, 5. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; El Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Yamani, H.A.; Pang, E.C.; Mantri, N.; Deighton, M.A. Antimicrobial Activity of Tulsi (Ocimum Tenuiflorum) Essential Oil and Their Major Constituents against Three Species of Bacteria. Front. Microbiol. 2016, 7, 681. [Google Scholar] [CrossRef] [Green Version]
- Nieto, G. A Review on Applications and Uses of Thymus in the Food Industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef]
- Rahaiee, S.; Moini, S.; Hashemi, M.; Shojaosadati, S.A. Evaluation of Antioxidant Activities of Bioactive Compounds and Various Extracts Obtained from Saffron (Crocus sativus L.): A Review. J. Food Sci. Technol. 2015, 52, 1881–1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological Properties of Salvia Officinalis and Its Components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Clavel-Coibrié, E.; Sales, J.R.; da Silva, A.M.; Barroca, M.J.; Sousa, I.; Raymundo, A. Sarcocornia Perennis: A Salt Substitute in Savory Snacks. Foods 2021, 10, 3110. [Google Scholar] [CrossRef]
- Fierascu, I.; Dinu-Pirvu, C.E.; Fierascu, R.C.; Velescu, B.S.; Anuta, V.; Ortan, A.; Jinga, V. Phytochemical Profile and Biological Activities of Satureja Hortensis L.: A Review of the Last Decade. Molecules 2018, 23, 2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, J.R.; Camargo, S.E.A.; de Oliveira, L.D. Rosmarinus officinalis L. (Rosemary) as Therapeutic and Prophylactic Agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Liberal, Â.; Fernandes, Â.; Polyzos, N.; Petropoulos, S.A.; Dias, M.I.; Pinela, J.; Petrović, J.; Soković, M.; Ferreira, I.C.F.R.; Barros, L. Bioactive Properties and Phenolic Compound Profiles of Turnip-Rooted, Plain-Leafed and Curly-Leafed Parsley Cultivars. Molecules 2020, 25, 5606. [Google Scholar] [CrossRef]
- Atar, H.; Çölgeçen, H. Bioactive Compounds of Oregano Seeds. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2020; pp. 73–77. [Google Scholar]
- Klimek-Szczykutowicz, M.; Dziurka, M.; Blažević, I.; Đulović, A.; Granica, S.; Korona-Glowniak, I.; Ekiert, H.; Szopa, A. Phytochemical and Biological Activity Studies on Nasturtium officinale (Watercress) Microshoot Cultures Grown in RITA® Temporary Immersion Systems. Molecules 2020, 25, 5257. [Google Scholar] [CrossRef]
- Brown, N.; John, J.A.; Shahidi, F. Polyphenol Composition and Antioxidant Potential of Mint Leaves. Food Prod. Process. Nutr. 2019, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.; Saeed, F.; Ahmad, N.; Ahmad, A.; Afzaal, M.; Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Anjum, F.M. Biochemical Profile of Milk Thistle (Silybum Marianum L.) with Special Reference to Silymarin Content. Food Sci. Nutr. 2021, 9, 244–250. [Google Scholar] [CrossRef]
- Alejo-Armijo, A.; Altarejos, J.; Salido, S. Phytochemicals and Biological Activities of Laurel Tree (Laurus nobilis). Nat. Prod. Commun. 2017, 12, 1934578X1701200. [Google Scholar] [CrossRef] [Green Version]
- Aluyor, E.O.; Oboh, I.O. PRESERVATIVES | Traditional Preservatives—Vegetable Oils. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 137–140. [Google Scholar]
- Aćimović, M.; Pezo, L.; Zeremski, T.; Lončar, B.; Marjanović Jeromela, A.; Stanković Jeremic, J.; Cvetković, M.; Sikora, V.; Ignjatov, M. Weather Conditions Influence on Hyssop Essential Oil Quality. Processes 2021, 9, 1152. [Google Scholar] [CrossRef]
- Cui, N.; Zhang, L.; Quan, M.; Xu, J. Profile of the Main Bioactive Compounds and in Vitro Biological Activity of Different Solvent Extracts from Ginkgo Biloba Exocarp. RSC Adv. 2020, 10, 45105–45111. [Google Scholar] [CrossRef] [PubMed]
- Sander, L.C. Soxhlet Extractions. J. Res. Natl. Inst. Stand. Technol. 2017, 122, 4. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive Compounds and Biological Functions of Garlic (Allium Sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [Green Version]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum Vulgare Mill: A Review of Its Botany, Phytochemistry, Pharmacology, Contemporary Application, and Toxicology. Biomed Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef] [Green Version]
- Burapan, S.; Kim, M.; Paisooksantivatana, Y.; Eser, B.E.; Han, J. Thai Curcuma Species: Antioxidant and Bioactive Compounds. Foods 2020, 9, 1219. [Google Scholar] [CrossRef]
- Mohammad Azmin, S.N.H.; Abdul Manan, Z.; Wan Alwi, S.R.; Chua, L.S.; Mustaffa, A.A.; Yunus, N.A. Herbal Processing and Extraction Technologies. Sep. Purif. Rev. 2016, 45, 305–320. [Google Scholar] [CrossRef]
- Abubakar, A.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied Sci. 2020, 12, 1. [Google Scholar] [CrossRef]
- Ghazy, O.A.; Fouad, M.T.; Saleh, H.H.; Kholif, A.E.; Morsy, T.A. Ultrasound-Assisted Preparation of Anise Extract Nanoemulsion and Its Bioactivity against Different Pathogenic Bacteria. Food Chem. 2021, 341, 128259. [Google Scholar] [CrossRef]
- Mehta, N.S.J.; Kumar, P.; Verma, A.K.; Umaraw, P.; Khatkar, S.K.; Khatkar, A.B.; Pathak, D.; Kaka, U.; Sazili, A.Q. Ultrasound-Assisted Extraction and the Encapsulation of Bioactive Components for Food Applications. Foods 2022, 11, 2973. [Google Scholar] [CrossRef]
- Louie, K.B.; Kosina, S.M.; Hu, Y.; Otani, H.; de Raad, M.; Kuftin, A.N.; Mouncey, N.J.; Bowen, B.P.; Northen, T.R. Mass Spectrometry for Natural Product Discovery. In Comprehensive Natural Products III; Elsevier: Amsterdam, The Netherlands, 2020; pp. 263–306. [Google Scholar]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber Officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Jitan, S.; Alkhoori, S.A.; Yousef, L.F. Phenolic Acids From Plants: Extraction and Application to Human Health. Stud. Nat. Prod. Chem. 2018, 58, 389–417. [Google Scholar] [CrossRef]
- Mukherjee, P.K. Extraction and Other Downstream Procedures for Evaluation of Herbal Drugs. In Quality Control and Evaluation of Herbal Drugs; Elsevier: Amsterdam, The Netherlands, 2019; pp. 195–236. [Google Scholar]
- Veggi, P.C.; Martinez, J.; Meireles, M.A.A. Fundamentals of Microwave Extraction. In Microwave-Assisted Extraction for Bioactive Compounds; Springer: New York, NY, USA, 2012; pp. 15–52. [Google Scholar]
- Solaberrieta, I.; Jiménez, A.; Garrigós, M.C. Valorization of Aloe Vera Skin By-Products to Obtain Bioactive Compounds by Microwave-Assisted Extraction: Antioxidant Activity and Chemical Composition. Antioxidants 2022, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.J.; Garlapati, V.K.; Gujjala, L.K.S.; Banerjee, R. Technologies for Oil Extraction from Oilseeds and Oleaginous Microbes. In Three Phase Partitioning; Elsevier: Amsterdam, The Netherlands, 2021; pp. 243–266. [Google Scholar]
- Kataoka, H. Pharmaceutical Analysis | Sample Preparation. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 231–255. [Google Scholar]
- Llompart, M.; Garcia-Jares, C.; Celeiro, M.; Dagnac, T. Microwave-Assisted Extraction. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 67–77. [Google Scholar]
- Sparr Eskilsson, C.; Björklund, E. Analytical-Scale Microwave-Assisted Extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Debbabi, H.; El Mokni, R.; Majdoub, S.; Aliev, A.; Hammami, S. The Effect of Pressure on the Characteristics of Supercritical Carbon Dioxide Extracts from Calamintha nepeta Subsp. Nepeta. Biomed. Chromatogr. 2020, 34, e4871. [Google Scholar] [CrossRef]
- Misic, D.; Tadic, V.; Korzeniowska, M.; Nisavic, J.; Aksentijevic, K.; Kuzmanovic, J.; Zizovic, I. Supercritical Fluid Extraction of Celery and Parsley Fruit-Chemical Composition and Antibacterial Activity. Molecules 2020, 25, 3163. [Google Scholar] [CrossRef]
- Asghar, A.; Abdullah; Irshad, M.A.; Majeed, M. Elucidating the Therapeutic Potential of Nutraceuticals. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2016; pp. 231–270. [Google Scholar]
- Cámara, M.; de Cortes Sánchez-Mata, M.; Fernández-Ruiz, V.; Cámara, R.M.; Manzoor, S.; Caceres, J.O. Lycopene: A Review of Chemical and Biological Activity Related to Beneficial Health Effects. Stud. Nat. Prod. Chem. 2013, 40, 383–426. [Google Scholar] [CrossRef]
- Ozkan, K.; Bayram, Y.; Karasu, S.; Karadag, A.; Sagdic, O. Extraction of Bioactive Compounds from Saffron Species. In Saffron; Elsevier: Amsterdam, The Netherlands, 2021; pp. 99–141. [Google Scholar]
- Aramrueang, N.; Asavasanti, S.; Khanunthong, A. Leafy Vegetables. In Integrated Processing Technologies for Food and Agricultural By-Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 245–272. [Google Scholar]
- Ashraf, R.; Ghufran, S.; Akram, S.; Mushtaq, M.; Sultana, B. Cold Pressed Coriander (Coriandrum sativum L.) Seed Oil. In Cold Pressed Oils; Elsevier: Amsterdam, The Netherlands, 2020; pp. 345–356. [Google Scholar]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of Polyphenols From Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 243–259. [Google Scholar]
- Xi, J. Ultrahigh Pressure Extraction of Bioactive Compounds from Plants—A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1097–1106. [Google Scholar] [CrossRef]
- Jauregi, P.; Alvarez-Ossorio, C.; Bald, C.; Ibarruri, J.; Iñarra, B.; San Martin, D.; Zufia, J. Enzymatic Processes for the Production of Food Ingredients from Food Processing By-Products. In Value-Addition in Food Products and Processing Through Enzyme Technology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 83–100. [Google Scholar]
- Sahne, F.; Mohammadi, M.; Najafpour, G.D.; Moghadamnia, A.A. Enzyme-Assisted Ionic Liquid Extraction of Bioactive Compound from Turmeric (Curcuma longa L.): Isolation, Purification and Analysis of Curcumin. Ind. Crops Prod. 2017, 95, 686–694. [Google Scholar] [CrossRef]
- Malik, J.; Mandal, S.C. Extraction of Herbal Biomolecules. In Herbal Biomolecules in Healthcare Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 21–46. [Google Scholar]
- Zeng, X.; Zhang, Z. Pulsed Electric Field Assisted Extraction of Bioactive Compounds. In Advances in Food Processing Technology; Springer: Singapore, 2019; pp. 125–135. [Google Scholar]
- Gharaati Jahromi, S. Extraction Techniques of Phenolic Compounds from Plants. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019; pp. 3–20. [Google Scholar]
- Cieśla, Ł.; Moaddel, R. Comparison of Analytical Techniques for the Identification of Bioactive Compounds from Natural Products. Nat. Prod. Rep. 2016, 33, 1131–1145. [Google Scholar] [CrossRef] [Green Version]
- Jönsson, A.-S.; Trägårdh, G. Ultrafiltration Applications. Desalination 1990, 77, 135–179. [Google Scholar] [CrossRef]
- Hage, D.S.; Anguizola, J.A.; Bi, C.; Li, R.; Matsuda, R.; Papastavros, E.; Pfaunmiller, E.; Vargas, J.; Zheng, X. Pharmaceutical and Biomedical Applications of Affinity Chromatography: Recent Trends and Developments. J. Pharm. Biomed. Anal. 2012, 69, 93–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Moraes, M.C.; Vanzolini, K.L.; Cardoso, C.L.; Cass, Q.B. New Trends in LC Protein Ligand Screening. J. Pharm. Biomed. Anal. 2014, 87, 155–166. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, W. Immobilized Enzyme Reactor in On-Line LC and Its Application in Drug Screening. Chromatographia 2009, 69, 5–12. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Zhang, X.; Chang, R.; Li, X. Development of a Stationary Phase of Vascular Smooth Muscle Cell Membrane Chromatography and Its Chromatographic Affinity Characteristics. Chromatographia 2011, 73, 1065–1071. [Google Scholar] [CrossRef]
- Zhuo, R.; Liu, H.; Liu, N.; Wang, Y. Ligand Fishing: A Remarkable Strategy for Discovering Bioactive Compounds from Complex Mixture of Natural Products. Molecules 2016, 21, 1516. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S. Dietary Supplements Market in India Is Rapidly Growing—An Overview. IMS Manag. J. 2018, 10, 1–6. [Google Scholar]
- Chopra, A.S.; Lordan, R.; Horbańczuk, O.K.; Atanasov, A.G.; Chopra, I.; Horbańczuk, J.O.; Jóźwik, A.; Huang, L.; Pirgozliev, V.; Banach, M.; et al. The Current Use and Evolving Landscape of Nutraceuticals. Pharmacol. Res. 2022, 175, 106001. [Google Scholar] [CrossRef]
- Kanchan Devendra, B.; Ravsaheb, M.S. A Review on Nutraceutical. Int. J. Res. Publ. Rev. 2022, 3, 3891–3899. [Google Scholar]
- Rubió, L.; Motilva, M.-J.; Romero, M.-P. Recent Advances in Biologically Active Compounds in Herbs and Spices: A Review of the Most Effective Antioxidant and Anti-Inflammatory Active Principles. Crit. Rev. Food Sci. Nutr. 2013, 53, 943–953. [Google Scholar] [CrossRef]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New Concepts in Nutraceuticals as Alternative for Pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar] [PubMed]
- Ohno, T.; Kato, N.; Ishii, C.; Shimizu, M.; Ito, Y.; Tomono, S.; Kawazu, S. Genistein Augments Cyclic Adenosine 3′5′-Monophosphate (CAMP) Accumulation and Insulin Release in Min6 Cells. Endocr. Res. 1993, 19, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Villa, P.; Costantini, B.; Suriano, R.; Perri, C.; Macrì, F.; Ricciardi, L.; Panunzi, S.; Lanzone, A. The Differential Effect of the Phytoestrogen Genistein on Cardiovascular Risk Factors in Postmenopausal Women: Relationship with the Metabolic Status. J. Clin. Endocrinol. Metab. 2009, 94, 552–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, G.; Xu, B.; Yao, D.; Zhang, D. Effect of Grape Seed Proanthocyanidin Extracts on Blood Glucose of Diabetic Mice. Nat. Prod. Res. Dev. 2012, 23, 1191. [Google Scholar]
- Li, X.; Liu, J.; Chang, Q.; Zhou, Z.; Han, R.; Liang, Z. Antioxidant and Antidiabetic Activity of Proanthocyanidins from Fagopyrum Dibotrys. Molecules 2021, 26, 2417. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.B.; Jørgensen, M.; Kotowska, D.; Petersen, R.K.; Kristiansen, K.; Christensen, L.P. Activation of the Nuclear Receptor PPARγ by Metabolites Isolated from Sage (Salvia officinalis L.). J. Ethnopharmacol. 2010, 132, 127–133. [Google Scholar] [CrossRef]
- Diab, F.; Zbeeb, H.; Baldini, F.; Portincasa, P.; Khalil, M.; Vergani, L. The Potential of Lamiaceae Herbs for Mitigation of Overweight, Obesity, and Fatty Liver: Studies and Perspectives. Molecules 2022, 27, 5043. [Google Scholar] [CrossRef]
- Nijhawan, P.; Behl, T. Nutraceuticals in the Management of Obesity. Obes. Med. 2020, 17, 100168. [Google Scholar] [CrossRef]
- Ninomiya, K.; Matsuda, H.; Shimoda, H.; Nishida, N.; Kasajima, N.; Yoshino, T.; Morikawa, T.; Yoshikawa, M. Carnosic Acid, a New Class of Lipid Absorption Inhibitor from Sage. Bioorg. Med. Chem. Lett. 2004, 14, 1943–1946. [Google Scholar] [CrossRef]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin Induces Browning of White Adipose Tissue and Counters Obesity by Activating TRPV1 Channel-Dependent Mechanisms. Br. J. Pharmacol. 2016, 173, 2369–2389. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.-H.; Tsuyoshi, G.; Han, I.-S.; Kawada, T.; Kim, Y.M.; Yu, R. Dietary Capsaicin Reduces Obesity-Induced Insulin Resistance and Hepatic Steatosis in Obese Mice Fed a High-Fat Diet. Obesity 2010, 18, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Chantre, P.; Lairon, D. Recent Findings of Green Tea Extract AR25 (Exolise) and Its Activity for the Treatment of Obesity. Phytomedicine 2002, 9, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-S.; Lee, J.H.; Ho, C.-T.; Bin Liu, C.; Wang, J.-M.; Wang, Y.-J.; Pan, M.-H. Rosmanol Potently Inhibits Lipopolysaccharide-Induced INOS and COX-2 Expression through Downregulating MAPK, NF-ΚB, STAT3 and C/EBP Signaling Pathways. J. Agric. Food Chem. 2009, 57, 10990–10998. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sung, B. Pharmacological Basis for the Role of Curcumin in Chronic Diseases: An Age-Old Spice with Modern Targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef]
- Ocaña-Fuentes, A.; Arranz-Gutiérrez, E.; Señorans, F.J.; Reglero, G. Supercritical Fluid Extraction of Oregano (Origanum vulgare) Essentials Oils: Anti-Inflammatory Properties Based on Cytokine Response on THP-1 Macrophages. Food Chem. Toxicol. 2010, 48, 1568–1575. [Google Scholar] [CrossRef]
- Savelev, S.; Okello, E.; Perry, N.S.L.; Wilkins, R.M.; Perry, E.K. Synergistic and Antagonistic Interactions of Anticholinesterase Terpenoids in Salvia Lavandulaefolia Essential Oil. Pharmacol. Biochem. Behav. 2003, 75, 661–668. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Salvia Officinalis Extract in the Treatment of Patients with Mild to Moderate Alzheimer’s Disease: A Double Blind, Randomized and Placebo-Controlled Trial. J. Clin. Pharm. Ther. 2003, 28, 53–59. [Google Scholar] [CrossRef]
- Howes, M.-J.R.; Houghton, P.J. Traditional Medicine for Memory Enhancement. In Herbal Drugs: Ethnomedicine to Modern Medicine; Springer: Berlin/Heidelberg, Germany, 2009; pp. 239–291. [Google Scholar]
- Eskelinen, M.H.; Ngandu, T.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Midlife Coffee and Tea Drinking and the Risk of Late-Life Dementia: A Population-Based CAIDE Study. J. Alzheimer’s Dis. 2009, 16, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Nyakas, C.; Felszeghy, K.; Szabó, R.; Keijser, J.N.; Luiten, P.G.M.; Szombathelyi, Z.; Tihanyi, K. Neuroprotective Effects of Vinpocetine and Its Major Metabolite Cis -Apovincaminic Acid on NMDA-Induced Neurotoxicity in a Rat Entorhinal Cortex Lesion Model. CNS Neurosci. Ther. 2009, 15, 89–99. [Google Scholar] [CrossRef]
- Inuwa, I.; Ali, B.H.; Al-Lawati, I.; Beegam, S.; Ziada, A.; Blunden, G. Long-Term Ingestion of Hibiscus Sabdariffa Calyx Extract Enhances Myocardial Capillarization in the Spontaneously Hypertensive Rat. Exp. Biol. Med. 2012, 237, 563–569. [Google Scholar] [CrossRef]
- Azimi, P.; Ghiasvand, R.; Feizi, A.; Hosseinzadeh, J.; Bahreynian, M.; Hariri, M.; Khosravi-Boroujeni, H. Effect of Cinnamon, Cardamom, Saffron and Ginger Consumption on Blood Pressure and a Marker of Endothelial Function in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Clinical Trial. Blood Press. 2016, 25, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, R.N.; Fatemeh Roozbeh Saravi, M.; Pourumir, M.; Jalali, F.; Moghadamnia, A.A. Investigation of the Effect of Ginger on the Lipid Levels. A Double Blind Controlled Clinical Trial. Saudi Med. J. 2008, 29, 1280–1284. [Google Scholar]
- Shelly, T.E.; McInnis, D.O.; Pahio, E.; Edu, J. Aromatherapy in the Mediterranean Fruit Fly (Diptera: Tephritidae): Sterile Males Exposed to Ginger Root Oil in Prerelease Storage Boxes Display Increased Mating Competitiveness in Field-Cage Trials. J. Econ. Entomol. 2004, 97, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yuan, C.; Wang, L.; Chen, R.; Li, X.; Zhang, Y.; Liu, C.; Liu, X.; Liang, W.; Xing, Y. The Beneficial Effects of Saffron Extract on Potential Oxidative Stress in Cardiovascular Diseases. Oxid. Med. Cell Longev. 2021, 2021, 6699821. [Google Scholar] [CrossRef]
- Nasiri, Z.; Sameni, H.R.; Vakili, A.; Jarrahi, M.; Khorasani, M.Z. Dietary Saffron Reduced the Blood Pressure and Prevented Remodeling of the Aorta in L-NAME-Induced Hypertensive Rats. Iran. J. Basic Med. Sci. 2015, 18, 1143–1146. [Google Scholar]
- Preuss, H.G.; Echard, B.; Polansky, M.M.; Anderson, R. Whole Cinnamon and Aqueous Extracts Ameliorate Sucrose-Induced Blood Pressure Elevations in Spontaneously Hypertensive Rats. J. Am. Coll. Nutr. 2006, 25, 144–150. [Google Scholar] [CrossRef]
- Akilen, R.; Tsiami, A.; Devendra, D.; Robinson, N. Glycated Haemoglobin and Blood Pressure-Lowering Effect of Cinnamon in Multi-Ethnic Type 2 Diabetic Patients in the UK: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Diabet. Med. 2010, 27, 1159–1167. [Google Scholar] [CrossRef]
- Ukoha, P.O.; Cemaluk, E.A.C.; Nnamdi, O.L.; Madus, E.P. Tannins and Other Phytochemical of the Samanaea Saman Pods and Their Antimicrobial Activities. Afr. J. Pure Appl. Chem. 2011, 5, 237–244. [Google Scholar]
- Faggian, M.; Bernabè, G.; Ferrari, S.; Francescato, S.; Baratto, G.; Castagliuolo, I.; Dall’Acqua, S.; Peron, G. Polyphenol-Rich Larix Decidua Bark Extract with Antimicrobial Activity against Respiratory-Tract Pathogens: A Novel Bioactive Ingredient with Potential Pharmaceutical and Nutraceutical Applications. Antibiotics 2021, 10, 789. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; D’Angelo, A.; Di Pierro, F. A Role for Quercetin in Coronavirus Disease 2019 (COVID-19). Phyther. Res. 2021, 35, 1230–1236. [Google Scholar] [CrossRef]
- Chanda, S.; Kaneria, M. Indian Nutraceutical Plant Leaves as a Potential Source of Natural Antimicrobial Agents. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; FORMATEX Research Center: Badajoz, Spain, 2011; pp. 1251–1259. [Google Scholar]
- Al Maqtari, M.A.A.; Alghalibi, S.M.; Alhamzy, E.H. Chemical Composition and Antimicrobial Activity of Essential Oil of Thymus Vulgaris from Yemen. Türk. Biyokim. Derg. Turkish J. Biochem. 2011, 36, 342–349. [Google Scholar]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heber, D.; Yip, I.; Ashley, J.M.; Elashoff, D.A.; Elashoff, R.M.; Go, V.L.W. Cholesterol-Lowering Effects of a Proprietary Chinese Red-Yeast-Rice Dietary Supplement. Am. J. Clin. Nutr. 1999, 69, 231–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brusq, J.-M.; Ancellin, N.; Grondin, P.; Guillard, R.; Martin, S.; Saintillan, Y.; Issandou, M. Inhibition of Lipid Synthesis through Activation of AMP Kinase: An Additional Mechanism for the Hypolipidemic Effects of Berberine. J. Lipid Res. 2006, 47, 1281–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.W.; Johnstone, B.M.; Cook-Newell, M.E. Meta-Analysis of the Effects of Soy Protein Intake on Serum Lipids. N. Engl. J. Med. 1995, 333, 276–282. [Google Scholar] [CrossRef]
- Saraheni, S.; David, W. Effect of Herbal Drink Plants Tiwai (Eleutherine Americana Merr) on Lipid Profile of Hypercholesterolemia Patients. Int. Food Res. J. 2014, 21, 1163–1167. [Google Scholar]
- Del Gobbo, L.C.; Falk, M.C.; Feldman, R.; Lewis, K.; Mozaffarian, D. Effects of Tree Nuts on Blood Lipids, Apolipoproteins, and Blood Pressure: Systematic Review, Meta-Analysis, and Dose-Response of 61 Controlled Intervention Trials. Am. J. Clin. Nutr. 2015, 102, 1347–1356. [Google Scholar] [CrossRef] [Green Version]
- Satia, J.A.; Littman, A.; Slatore, C.G.; Galanko, J.A.; White, E. Associations of Herbal and Specialty Supplements with Lung and Colorectal Cancer Risk in the VITamins And Lifestyle Study. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 1419–1428. [Google Scholar] [CrossRef] [Green Version]
- Phua, D.H.; Zosel, A.; Heard, K. Dietary Supplements and Herbal Medicine Toxicities—When to Anticipate Them and How to Manage Them. Int. J. Emerg. Med. 2009, 2, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Hudson, A.; Lopez, E.; Almalki, A.J.; Roe, A.L.; Calderón, A.I. A Review of the Toxicity of Compounds Found in Herbal Dietary Supplements. Planta Med. 2018, 84, 613–626. [Google Scholar] [CrossRef] [Green Version]
- Navarro, V.; Lucena, M. Hepatotoxicity Induced by Herbal and Dietary Supplements. Semin. Liver Dis. 2014, 34, 172–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, D.W.; Segall, H.J.; Pan, L.C.; Lamé, M.W.; Estep, J.E.; Morin, D. Mechanisms and Pathology of Monocrotaline Pulmonary Toxicity. Crit. Rev. Toxicol. 1992, 22, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, S.J.P.L.; Restani, P.; Boersma, M.G.; Delmulle, L.; Rietjens, I.M.C.M. Levels of Genotoxic and Carcinogenic Ingredients in Plant Food Supplements and Associated Risk Assessment. Food Nutr. Sci. 2011, 02, 989–1010. [Google Scholar] [CrossRef] [Green Version]
- Mei, N.; Guo, L.; Fu, P.P.; Fuscoe, J.C.; Luan, Y.; Chen, T. Metabolism, Genotoxicity, Annd Carcinogenicity of Comfrey. J. Toxicol. Environ. Health Part B 2010, 13, 509–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiliś-Pstrusińska, K.; Wiela-Hojeńska, A. Nephrotoxicity of Herbal Products in Europe—A Review of an Underestimated Problem. Int. J. Mol. Sci. 2021, 22, 4132. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A.; Ernst, E. Safety of Herbal Supplements: A Guide for Cardiologists. Cardiovasc. Ther. 2010, 28, 246–253. [Google Scholar] [CrossRef]
- Thakkar, S.; Anklam, E.; Xu, A.; Ulberth, F.; Li, J.; Li, B.; Hugas, M.; Sarma, N.; Crerar, S.; Swift, S.; et al. Regulatory Landscape of Dietary Supplements and Herbal Medicines from a Global Perspective. Regul. Toxicol. Pharmacol. 2020, 114, 104647. [Google Scholar] [CrossRef]
- Williams, C.T. Herbal Supplements. Nurs. Clin. N. Am. 2021, 56, 1–21. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Reddy, K.R. Review Article: Herbal and Dietary Supplement Hepatotoxicity. Aliment. Pharmacol. Ther. 2013, 37, 3–17. [Google Scholar] [CrossRef]
- De Boer, Y.S.; Sherker, A.H. Herbal and Dietary Supplement–Induced Liver Injury. Clin. Liver Dis. 2017, 21, 135–149. [Google Scholar] [CrossRef] [Green Version]
- Ko, R. Safety of Ethnic & Imported Herbal and Dietary Supplements. Clin. Toxicol. 2006, 44, 611–616. [Google Scholar] [CrossRef] [PubMed]


| Herbs | Scientific Name | Bioactive Compound | Functions | References |
|---|---|---|---|---|
| Aloe vera | Aloe barbadensis Miller. | Flavonoids, lectins, terpenoids, fatty acids, tannins, anthraquinones, pectins, hemicelluloses, glucomannan, campesterol, β-sitosterol, salicylic acid and vitamins, such as A, C, E, β-carotene, B1, B2, B3, B6, choline, B12, folic acid | Anti-inflammatory properties, treatment of skin problems, such as wounds burns; antihyperlipidemic, anticancer, antidiabetic and antioxidant properties. | [3,4] |
| Angelica | Angelica archangelica. | β-phellandrene, umbelliprenin, phenols, furocoumarins, such as bergapten, xanthotoxin and angelicin | Treatment of arthritis, heartburn, flatulence, anorexia, circulation problems, respiratory catarrh, insomnia, nervousness and plague. | [3,5] |
| Anise | Pimpinella anisum. | Trans-anethole, coumarins, flavonoids and lipids | Treatment of constipation, indigestion, menopausal problems and migraine; also protects against insects. | [3,6] |
| Aralia | Polyscias fruticose. | Petroselinic acid, triterpenoid saponins, sterols, diterpenoids, and acetylenic lipids | Treatment of rheumatoid arthritis, hepatitis bruises, carbuncles, and lumps. | [2,3] |
| Bay | Laurus nobilis. L. | Cholesteric-7-en-3β-ol, cholesteric-4-en-3β,6β-diol, batilol and ceramide | Regulation of uric acid levels and blood cholesterol; also elicits anti-inflammatory, antidiarrheal and antidiabetic activities | [3,7] |
| Bayberry | Myrica pensylvanica. | Anthocyanidins and flavonols | Treatment of sore throat, vaginal discharge, colitis, wounds, ulcers, headache, colds, nausea and diarrhea; also enhances circulation. | [3,8] |
| Bee balm | Monarda didyma L. | Polyphenols, flavonoids, monoterpenoid aldehyde, monoterpene glycosides, triterpenes, sesquiterpenes, resin, tannin and essential oils | Elicits diaphoretic, antiseptic, emmenagogue, antimicrobial, antispasmodic, anti-inflammatory actions; may also acts as a carminative, diuretic, expectorant, and sedative. | [1,3] |
| Burnet | Sanguisorba minor. | Phenolic acids (chlorogenic, ellagic, gallic, caffeic, and rosmarinic acid), flavonoids, catechin derivatives (catechin, epigallocatechin gallate) and neolignans | Treatment of ulcerative colitis, dysentery, diarrhea, bladder problems, hemorrhoids, phlebitis and varicose veins. | [3,9] |
| Calamint | Clinopodium nepeta. | Dihydrocarveol, dihydrocarveol acetate, dihydrocarveol, 1,8 cineole, cis-carvyl acetate, and pulegone | Shows antioxidant, antimicrobial, anti-ulcer, anti-inflammatory, insecticidal properties. | [3,9] |
| Caraway | Carum carvi. | Carvacrol, carvone, α-pinene, limonene, γ-terpinene, linalool, carvenone, and p-cymene | It acts as an expectorant, stimulant and antispasmodic agent; can be used to treat nausea, stomach aches and constipation. | [3,10] |
| Chamomile | Matricaria recutita. | Chamomile are levomenol and its oxides, apigenin, azulenes, farnesene, spathuleno, and spiroethers | Treatment of hay fever, muscle spasms, inflammation, menstrual disorders, wounds, ulcers, insomnia, gastrointestinal disorders, hemorrhoids and rheumatic pain. | [3,11] |
| Dill | Anethum graveolens L. | Sinapic, vanillic acids and rutin | Has been traditionally used for treating stomach ailments, colic pain, hiccups, bad breath, flatulence, and hemorrhoids. | [3,12] |
| Fennel | Foeniculum vulgare. | Quinic acid, 4-O-caffeoylquinic acid, p-coumaric acid, and 4-O-caffeoylquinic acid, rosmarinic acid and chlorogenic acids | Beneficial actions on immune system, collagen synthesis, tissue repair, cellular protection, blood sugar regulation, bone development, and wound healing. | [3,34] |
| Garlic | Allium sativum. | Diallyl thiosulfonate (allicin), diallyl sulfide, diallyl disulfide, diallyl trisulfide, E/Z-ajoene, S-allyl-cysteine, and S-allyl-cysteine sulfoxide (L-alliin) | Decreases the risk of cancers, and osteoarthritis; may treat cardiovascular diseases, elevated cholesterol and blood pressure. | [3,33] |
| Ginger | Zingiber officinale. | Gingerols, shogaols, and paradols | Treatment of indigestion, nausea, flu, regulates blood insulin levels and BMI | [3,32] |
| Gingko | Ginkgo biloba. | Terpenoids, flavonoids, biflavonoids, organic acids, polyprenols, ginkgolides and bilobalide | Enhancement of memory and can treat blood disorders. | [3,31] |
| Hyssop | Hyssopus officinalis. | Diosmin, isopinocamphone and pinocamphone | Treatment of toothache, and dysfunctions of the nervous, pulmonary, uterine, digestive and urinary systems. | [3,30] |
| Lemon grass | Cymbopogon citratus. | Myrcene, limonene, citral, geraniol, citronellol, geranyl acetate, neral, and nerol | Reduces pain, fever, blood sugar and cholesterol; enhances menstrual flow and acts as an antioxidant. | [3,29] |
| Laurel | Laurus nobilis. | Cinnamtannin B-1, trimeric A-type procyanidin, polyphenolic compounds, alkaloids, norisoprenoids, sugars, polysaccharides, organic acids and tocopherols | Treatment of rheumatism, cardiac diseases, cough, viral infections, and diarrhea; enhances gastric secretion and has diaphoretic and antiseptic activity. | [3,35] |
| Milk thistle | Silybum marianum. | Apigenin, silybonol, betaine, free fatty acids, silybin, silychristin and silidianin | Treatment of hepatitis, cirrhosis, jaundice, diabetes, and indigestion. | [3,27] |
| Mint | Mentha piperita L. | Eriocitrin, rosmarinic acid, luteolin 7-O-rutinoside, hesperidin, caffeic acid, ferulic acid, eugenol, pebrellin, gardenin B and apigenin | Has antiseptic and antibacterial properties and is used to treat digestive problems. | [3,26] |
| Nasturtium | Tropaeolum majus. | Flavonoids, glucosinolates, anthocyanin, and fatty acids | Strong antiseptic properties and used in treatment of wounds and fungal infections; vapors may treat bronchitis and other lung infections. | [3,25] |
| Oregano | Origanum vulgare. | carvacrol, β-fenchyl alcohol, thymol, and γ-terpinene, phenolic compounds, flavonoids, flavanones, tocopherols, carvacrol, benzoic acid, rosmarinic acid, and cinnamic acid derivatives | Treatment of asthma, cough, diarrhea, stomachache, sores, muscle aches and menstrual inflammatory disorders. | [3,24] |
| Parsley | Petroselinum crispum. | Furanocoumarins, essentials oils, flavonoids, carotenoids, vitamins, minerals (e.g., iron, zinc, calcium and phosphorous) and fatty acids | Acts as a diuretic and decreases bloating; regulates blood pressure; vitamin K stimulates bone growth and increases bone density. | [3,23] |
| Rosemary | Salvia rosmarinus. | Caffeic acid, carnosic acid, chlorogenic acid, monomeric acid, oleanolic acid, rosmarinic acid, ursolic acid, alpha-pinene, camphor, carnosol, eucalyptol, rosmadial, rosmanol, rosmaquinones A and B, secohinokio, and derivatives of eugenol and luteolin | Treatment of muscle pain; boosts memory, circulatory system and immune system; and promotes hair growth. | [3,22] |
| Savory | Satureja hortensis L. | Coumarin, phenolic acids, hydroxybenzoic acids, flavonoids, linoleic acid, oleic acid, phytosterols, and pectic polysaccharides | Treatment of cramps, diarrhea, nausea, indigestion and intestinal gas; reduces cough, sore throat and sex drive. | [3,21] |
| Sage | Salvia officinalis. | Borneol, camphor, caryophyllene, cineole, elemene, humulene, ledene, pinene, and thujone, rosmarinic acid and luteolin-7-glucoside, caffeic acid and 3-Caffeoylquinic acid, chlorogenic acid, ellagic acid, epicatechin, epigallocatechin gallate, quercetin, rosmarinic acid, rutin, and luteolin-7-glucoside, borneol, cineole, camphor, and thujone, Rosmarinic acid and ellagic acid, rutin, chlorogenic acid, and quercetin | Treatment of ulcers, seizures, gout, rheumatism, inflammation, tremor, dizziness, paralysis, diarrhea and hyperglycemia. | [3,20] |
| Saffron | Crocus sativus L. | Crocin, crocetin, carotene, safranal and picrocrocin | Treatment of libido; boosts mood and memory. | [3,19] |
| Thyme | Thymus vulgaris. | Carvacrol, thymol, ρ-cimeno monoterpene hydrocarbons, γ-terpinen, linoleic, oleic, stearic, and palmitic, γ-Tocopherol and α-tocotrienol | Reduces acne, blood pressure, and cough; also enhance immunity and act as a pest repellent. | [3,18] |
| Tulsi | Ocimum tenuiflorum. | Ursolic acid, eugenol, rosmarinic acid, linalool, carvacrol, β caryophyllene, oleanolic acid, ursolic acid, rosmarinic acid | Exhibits antimicrobial, antidiabetic, adaptogenic, hepatoprotective, anticarcinogenic, anti-inflammatory, radioprotective, neuroprotective immunomodulator, cardioprotective actions. | [3,17] |
| Turmeric | Curcuma longa. | Curcumin, curcumin II [demethoxycurcumin, 1-(4-hydroxy-3-methoxyphenyl)-7-(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione] and curcumin III [bisdemethoxycurcumin, 1,7-bis(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione] | Elevation of overall energy of the body; dispels worms, regulates menstruation, improves digestion and relieves arthritis. | [3,16] |
| Valerian | Valeriana officinalis. | Non-glycosidic iridoid esters, valepotriates and flavonoids | Treatment of headaches, depression, insomnia, anxiety, premenstrual syndrome (PMS), and menopause symptoms. | [3,15] |
| Wormwood | Artemisia absinthium L. | Dimeric guaianolides–absinthins, monoterpene hydrocarbons–chamazulene | Treatment of upset stomach, loss of appetite, gall bladder dysfunction, liver diseases and intestinal spasms; can be used to treat fever, worm infections and increase sexual desire. | [3,14] |
| Yarrow | Achillea millefolium. | Azulene, caryophyllene, thujone, eucalyptol, α- and β-pinene and borneol, as well as lactones, tannins and alkaloids | Treatment of hay fever, common cold, menstruation problems, diarrhea, dysentery, loss of appetite, and gastric discomfort; induces sweating. | [2,3] |
| Zedoary | Curcuma zedoaria. | Curcumin, demethoxycurcumin, bisdemethoxycurcumin, 1,7-diphenyl-(4E,6E)-4,6-heptadien-3-ol, germacrone, furanodienone, zederone, and ar-turmerone | Treatment of indigestion, spasms and loss of appetite, fatigue, anxiety, stress, pain and inflammation. | [3,13] |
| Product Name | Form | Herbal Ingredients Used | Benefits | Manufacturer |
|---|---|---|---|---|
| Aloe vera | Powder | Aloe vera | Anti-viral, anti-bacterial and anti-inflammatory | Purenso Select |
| 200x | ||||
| Amla powder | Powder | Emblica officinalis | Antioxidant, enhances cardiac health and decreases levels of bad cholesterol, offers a rise in immunity and boosts energy in a frail body | Bixa Botanical |
| Ayurvedic hair and vitality booster juice | Syrup | Winter cherry, false daisy, spikenard | Helps men tackle issues, such as decreased energy levels and recurring hair problems | Sesa Care Pvt. Ltd. |
| Bramhi | Pill | Water hyssop | Increases and improves intelligence, stimulates mental agility and treats weak memory | Himalaya Wellness Company |
| Nutrilite soft gels | Gel | Green tea extract | Positively affects skin health | Amway |
| De-stress | Syrup | Water hyssop, spikenard, winter cherry, ginseng, aloe-weed | Relieves from sleep and stress related issues | Naturamore |
| Dr noni juice | Syrup | Garcinia | Helps in joint pains, maintain a healthy heart, aids in maintaining healthy blood sugar levels, stimulates the release of digestive enzymes | LA Nutraceuticals |
| Fenugreek tablets | Pill | Fenugreek | Enhances immunity, stimulates lactation and improves wellness | Bhumija Lifesciences |
| Ginkgo biloba, bilberry, and lutein | Capsule | Ginkgo biloba, bilberry, lutein | Boosts blood flow to the brain, which may be beneficial for depression, headaches, vertigo, tinnitus, and memory loss; also enhances eyesight. | Healthvit |
| Go365 nutra tablet | Pill | Turmeric and Boswellia | Reduces inflammation and prevents cartilage degradation, reduces chronic joint pain, stiffness, and swelling | Charak Pharma Pvt. Ltd. |
| Hair skin vitamins | Capsule | Turmeric and primrose oil, hyaluronic acid | Stimulates hair strength and glowing skin | Neuherbs |
| Herbalance | Gel | Chasteberry, Rhodiola rosea, red raspberry | Aids in maintaining hormonal balance and treats menstrual problems | Oziva |
| Holistic calcium and curcumin | Pill | Curcuminoids and piperine | Fights cancer, ageing, arthritis and inflammation | Zeroharm Sciences |
| Joint-fit 90N | Capsule | Nut grass, winter cherry, holy basil, moonseed | Maintains healthy function of joints and muscles, reduces chronic inflammation | Organic Wellness |
| Long looks Capsules | Capsule | Vitex negundo, moonplant, Cassia tora, Acacia nilotica | Stimulates optimal physical growth, enhances bone growth | Orgovedic |
| Numoringo juice | Syrup | Moringa oleifera, Oregano vulgare, Piper nigrum | Regulates blood pressure, joint functions, enhances endurance and aids digestion | Hawaiian Herbal |
| Organic spinach | Capsule | Spinach | Elicits antioxidant activity and boosts immunity, promotes eye, skin, bone and heart health, aids in weight loss and ameliorates anemia | Xovak Pharma |
| Organic Tribulus terrestris powder | Powder | Tribulus terrestris | Testosterone and muscle booster | Herbadiet |
| Plump up Anti wrinkle gel | Gel | Winter cherry, grapeseed and fennel | Protects against environmental and sun-induced skin problems | Just Herbs |
| Rose and aloe vera facial massage gel | Gel | Aloe vera, Azadirachta indica and basil | Wound healing and anti-inflammatory property | Khadi Natural Healthcare |
| Sat isabgol | Powder | Psyllium from Plantago ovata | Controls cholesterol levels and promotes digestion and laxation. | Sidhapur Sat-Isabgol Factory |
| Spirulina green food supplement | Pill | Spirulina | Antioxidant functions, promotes metabolism, increases strength and boosts immunity | Ayur Champ |
| Steel-libido peak testosterone | Gel | Winter cherry extract | Peak performance enhancer and testosterone booster | Irwin Naturals |
| Upakarma ayurveda ginger drops | Syrup | Ginger | Boosts immunity and strength | Upakarma Ayurveda |
| Yakrit plihantak churna | Powder | Phyllanthus niruri, Eclipta alba, Andrographis paniculate, Tephrosia purpurea, Cichorium intybus | Supports healthy liver and spleen, maintains overall health and well being | Planet Ayurveda |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bommakanti, V.; Puthenparambil Ajikumar, A.; Sivi, C.M.; Prakash, G.; Mundanat, A.S.; Ahmad, F.; Haque, S.; Prieto, M.A.; Rana, S.S. An Overview of Herbal Nutraceuticals, Their Extraction, Formulation, Therapeutic Effects and Potential Toxicity. Separations 2023, 10, 177. https://doi.org/10.3390/separations10030177
Bommakanti V, Puthenparambil Ajikumar A, Sivi CM, Prakash G, Mundanat AS, Ahmad F, Haque S, Prieto MA, Rana SS. An Overview of Herbal Nutraceuticals, Their Extraction, Formulation, Therapeutic Effects and Potential Toxicity. Separations. 2023; 10(3):177. https://doi.org/10.3390/separations10030177
Chicago/Turabian StyleBommakanti, Vaishnavi, Amruthamol Puthenparambil Ajikumar, Chelssa Maria Sivi, Geethika Prakash, Anjaly Shanker Mundanat, Faraz Ahmad, Shafiul Haque, Miguel Angel Prieto, and Sandeep Singh Rana. 2023. "An Overview of Herbal Nutraceuticals, Their Extraction, Formulation, Therapeutic Effects and Potential Toxicity" Separations 10, no. 3: 177. https://doi.org/10.3390/separations10030177
APA StyleBommakanti, V., Puthenparambil Ajikumar, A., Sivi, C. M., Prakash, G., Mundanat, A. S., Ahmad, F., Haque, S., Prieto, M. A., & Rana, S. S. (2023). An Overview of Herbal Nutraceuticals, Their Extraction, Formulation, Therapeutic Effects and Potential Toxicity. Separations, 10(3), 177. https://doi.org/10.3390/separations10030177