Unveiling Novel Chaotropic Chromatography Method for Determination of Pralidoxime in Nerve Agent Antidote Autoinjectors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Preparation of Standard Solutions and Sample
2.3. Instruments and Equipment
2.4. Chromatographic Conditions
3. Results and Discussion
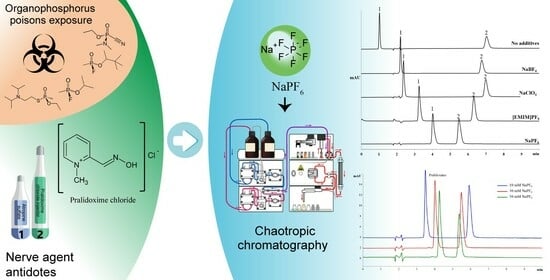
3.1. Advancing Pralidoxime Separation Using Chaotropic Salt as a Mobile-Phase Modifier
3.2. Optimizing Chaotrope Concentrations for Enhanced Chromatographic Separation of Pralidoxime
3.3. Comparison with Current Official Analytical Method of Pralidoxime in Pharmacopoeia
3.4. Method Validation
3.4.1. Linearity
3.4.2. Intra- and Inter-Day Accuracy and Precision
3.4.3. Recovery
3.4.4. Repeatability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pulkrabkova, L.; Svobodova, B.; Konecny, J.; Kobrlova, T.; Muckova, L.; Janousek, J.; Pejchal, J.; Korabecny, J.; Soukup, O. Neurotoxicity evoked by organophosphates and available countermeasures. Arch. Toxicol. 2023, 97, 39–72. [Google Scholar] [CrossRef]
- Tang, X.; Wang, R.; Xie, H.; Hu, J.; Zhao, W. Repeated pulse intramuscular injection of pralidoxime chloride in severe acute organophosphorus pesticide poisoning. Am. J. Emerg. Med. 2013, 31, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Pirollo, K.F.; Moghe, M.; Guan, M.; Rait, A.S.; Wang, A.; Kim, S.-S.; Chang, E.H.; Harford, J.B. A Pralidoxime Nanocomplex Formulation Targeting Transferrin Receptors for Reactivation of Brain Acetylcholinesterase After Exposure of Mice to an Anticholinesterase Organophosphate. Int. J. Nanomed. 2024, 19, 307–326. [Google Scholar] [CrossRef] [PubMed]
- John, H.; Blum, M.-M. Review of UV spectroscopic, chromatographic, and electrophoretic methods for the cholinesterase reactivating antidote pralidoxime (2-PAM). Drug Test. Anal. 2012, 4, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Antoniello, A.A.; Pauls, P.; Awad, N.I.; Sobolewski, K.; Fernández, D.; Bridgeman, P. Optimization of antidote stocking, availability, and administration practices for a large multihospital organization. Am. J. Health-Syst. Pharm. 2022, 80, S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Rawas-Qalaji, M.; Thu, H.E.; Hussain, Z. Chapter 23—Potential alternative treatments and routes of administrations: Nerve agents poisoning. In Sensing of Deadly Toxic Chemical Warfare Agents, Nerve Agent Simulants, and their Toxicological Aspects; Das, S., Thomas, S., Das, P.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 539–568. [Google Scholar]
- Chung, S.P.; Roh, H.-K. Antidote for organophosphate insecticide poisoning: Atropine and pralidoxime. Korean Med. Assoc. 2013, 56, 1057–1066. [Google Scholar] [CrossRef]
- Vijayaraghavan, R. Autoinjector device for rapid administration of drugs and antidotes in emergency situations and in mass casualty management. J. Int. Med. Res. 2020, 48, 0300060520926019. [Google Scholar] [CrossRef] [PubMed]
- Menke, B.A.; Ryu, C.; Justin, G.A.; Chundury, R.V.; Hayek, B.R.; Debiec, M.R.; Yeh, S. Ophthalmic manifestations and management considerations for emerging chemical threats. Front. Toxicol. 2023, 5, 1281041. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.C.; Digiovanni, J.H.; Von Bredow, J.; Heiffer, M.H. Pralidoxime chloride stability-indicating assay and analysis of solution samples stored at room temperature for ten years. J. Pharm. Sci. 1989, 78, 132–136. [Google Scholar] [CrossRef]
- Paddle, B.M.; Dowling, M.H. Simple high-performance liquid chromatographic method for assessing the deterioration of atropine-oxime mixtures employed as antidotes in the treatment of nerve agent poisoning. J. Chromatogr. 1993, 648, 373–380. [Google Scholar] [CrossRef]
- Bariakar, U.V.; Patel, U.N. Pralidoxime Chloride. In Analytical Profiles of Drug Substances; Academic Press: Cambridge, MA, USA, 1988; pp. 533–569. [Google Scholar]
- Utley, D. Determination of 2-hydroxyiminomethyl-1-methyl-pyridinium methanesulphonate (pralidoxime mesylate, P2S) and its degradation products in solution by liquid chromatography. J. Chromatogr. A 1983, 265, 311–322. [Google Scholar] [CrossRef]
- Prue, D.G.; Johnson, R.N.; Kho, B.T. High-performance liquid chromatographic determination of pralidoxime chloride and its major decomposition products in injectable solutions. J. Pharm. Sci. 1983, 72, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Ellin, R.I. Stability of concentrated aqueous solutions of pralidoxime chloride. J. Pharm. Sci. 1982, 71, 1057–1059. [Google Scholar] [CrossRef] [PubMed]
- Szegi, P.; Kalász, H.; Laufer, R.; Kuca, K.; Tekes, K. Pyridinium aldoxime analysis by HPLC: The method for studies on pharmacokinetics and stability. Anal. Bioanal. Chem. 2010, 397, 579–586. [Google Scholar] [CrossRef]
- Žuvela, P.; Skoczylas, M.; Jay Liu, J.; Bączek, T.; Kaliszan, R.; Wong, M.W.; Buszewski, B. Column Characterization and Selection Systems in Reversed-Phase High-Performance Liquid Chromatography. Chem. Rev. 2019, 119, 3674–3729. [Google Scholar] [CrossRef]
- Suh, J.H.; Kim, J.; Jung, J.; Kim, K.; Lee, S.G.; Cho, H.-D.; Jung, Y.; Han, S.B. Determination of thiamine in pharmaceutical preparations by reverse phase liquid chromatography without use of organic solvent. Bull. Korean Chem. Soc. 2013, 34, 1745–1750. [Google Scholar] [CrossRef]
- Zhao, S.; Li, L. Chemical derivatization in LC-MS-based metabolomics study. TrAC Trends Anal. Chem. 2020, 131, 115988. [Google Scholar] [CrossRef]
- Iturrospe, E.; Da Silva, K.M.; Talavera Andújar, B.; Cuykx, M.; Boeckmans, J.; Vanhaecke, T.; Covaci, A.; van Nuijs, A.L.N. An exploratory approach for an oriented development of an untargeted hydrophilic interaction liquid chromatography-mass spectrometry platform for polar metabolites in biological matrices. J. Chromatogr. A 2021, 1637, 461807. [Google Scholar] [CrossRef]
- Jandera, P.; Hájek, T. Dual-mode hydrophilic interaction normal phase and reversed phase liquid chromatography of polar compounds on a single column. J. Sep. Sci. 2020, 43, 70–86. [Google Scholar] [CrossRef]
- Axente, R.E.; Stan, M.; Chitescu, C.L.; Nitescu, V.G.; Vlasceanu, A.-M.; Baconi, D.L. Application of Ionic Liquids as Mobile Phase Additives for Simultaneous Analysis of Nicotine and Its Metabolite Cotinine in Human Plasma by HPLC–DAD. Molecules 2023, 28, 1563. [Google Scholar] [CrossRef] [PubMed]
- Goyon, A.; Blevins, M.S.; Napolitano, J.G.; Nguyen, D.; Goel, M.; Scott, B.; Wang, J.; Koenig, S.G.; Chen, T.; Zhang, K. Characterization of antisense oligonucleotide and guide ribonucleic acid diastereomers by hydrophilic interaction liquid chromatography coupled to mass spectrometry. J. Chromatogr. A 2023, 1708, 464327. [Google Scholar] [CrossRef] [PubMed]
- Violi, J.P.; Bishop, D.P.; Padula, M.P.; Steele, J.R.; Rodgers, K.J. Considerations for amino acid analysis by liquid chromatography-tandem mass spectrometry: A tutorial review. TrAC Trends Anal. Chem. 2020, 131, 116018. [Google Scholar] [CrossRef]
- Long, Z.; Guo, Z.; Xue, X.; Zhang, X.; Nordahl, L.; Liang, X. Selective separation and purification of highly polar basic compounds using a silica-based strong cation exchange stationary phase. Anal. Chim. Acta 2013, 804, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Abdulhussain, N.; Nawada, S.; Schoenmakers, P. Latest Trends on the Future of Three-Dimensional Separations in Chromatography. Chem. Rev. 2021, 121, 12016–12034. [Google Scholar] [CrossRef]
- Lu, J.; Xiong, X.; Ma, M.; Chen, B.; Chen, Y.; Schmitz, O.J. Enhancing the compatibility of normal-phase chromatography x reversed−phase chromatography by combination of low−temperature sensitive aqueous-phase compatible normal-phase chromatography and at−column dilution modulation. J. Chromatogr. A 2023, 1691, 463821. [Google Scholar] [CrossRef] [PubMed]
- Kartsova, L.A.; Makeeva, D.V.; Kravchenko, A.V.; Moskvichev, D.O.; Polikarpova, D.A. Capillary electrophoresis as a powerful tool for the analyses of bacterial samples. TrAC Trends Anal. Chem. 2021, 134, 116110. [Google Scholar] [CrossRef]
- Kumagai, M.; Kato, S.; Arakawa, N.; Otsuka, M.; Hamano, T.; Kashiwagi, N.; Yabuki, A.; Yamato, O. Quantification of Histidine-Containing Dipeptides in Dolphin Serum Using a Reversed-Phase Ion-Pair High-Performance Liquid Chromatography Method. Separations 2021, 8, 128. [Google Scholar] [CrossRef]
- Voicu, V.; Albu, F.; Tache, F.; Musilek, K.; Kuca, K.; Medvedovici, A. LC–MS/MS approaches for the assay of bis-quaternary pyridinium oximes used as AChE reactivators in biological matrices. Bioanalysis 2013, 5, 793–809. [Google Scholar] [CrossRef]
- Voicu, V.; Medvedovici, A.; Radulescu, M.; Iorgulescu, E.-E.; David, V. Unusual Retention Behavior of Some Cationic-Type Aldoximes Used as AChE Reactivators Under Ion-Pairing Liquid Chromatographic Mechanism. Anal. Lett. 2010, 43, 1267–1276. [Google Scholar] [CrossRef]
- USP monographs. Pralidoxime Chloride for Injection; USP-NF; Unites States Pharmacopoeia: Rockville, MD, USA, 2023. [Google Scholar]
- The Korean Pharmacopoeia, 12th ed.; The KFDA Notification: Cheongju, Republic of Korea, 2020.
- Protti, M.; Carotti, A.; Mercolini, L.; Sardella, R. Chapter 12—Co-solvents and mobile phase additives in HPLC. In Liquid Chromatography, 3rd ed.; Fanali, S., Chankvetadze, B., Haddad, P.R., Poole, C.F., Riekkola, M.-L., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, pp. 283–321. [Google Scholar]
- Dullinger, P.; Horinek, D. Solvation of Nanoions in Aqueous Solutions. J. Am. Chem. Soc. 2023, 145, 24922–24930. [Google Scholar] [CrossRef] [PubMed]
- Djajić, N.; Krmar, J.; Rmandić, M.; Rašević, M.; Otašević, B.; Zečević, M.; Malenović, A.; Protić, A. Modified aqueous mobile phases: A way to improve retention behavior of active pharmaceutical compounds and their impurities in liquid chromatography. J. Chromatogr. Open 2022, 2, 100023. [Google Scholar] [CrossRef]
- Aliyeva, M.; Brandão, P.; Coutinho, J.A.P.; Ferreira, O.; Pinho, S.P. Solubilities of Amino Acids in the Presence of Chaotropic Anions. J. Solut. Chem. 2023, 1–11. [Google Scholar] [CrossRef]
- Soares, B.P.; Abranches, D.O.; Sintra, T.E.; Leal-Duaso, A.; García, J.I.; Pires, E.; Shimizu, S.; Pinho, S.P.; Coutinho, J.A.P. Glycerol Ethers as Hydrotropes and Their Use to Enhance the Solubility of Phenolic Acids in Water. ACS Sustain. Chem. Eng. 2020, 8, 5742–5749. [Google Scholar] [CrossRef]
- Lazarevska Todevska, E.; Piponski, M.; Stefova, M. Development and validation of an analytical method for the determination of related substances in bisoprolol fumarate in dosage forms by HPLC-UV-DAD. Maced. J. Chem. Chem. Eng. 2021, 40, 263–276. [Google Scholar] [CrossRef]
- USP43-NF38-3637; Pralidoxime Chloride for Injection. USP-NF: Frederick, MD, USA, 2020.
- Hoffman, R.S.; Mercurio-Zappala, M.; Bouchard, N.; Ravikumar, P.; Goldfrank, L. Preparing for chemical terrorism: A study of the stability of expired pralidoxime (2-PAM). Disaster Med. Public Health Prep. 2011, 6, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Chattopadhyay, P.; Ghosh, A.; Kaity, S.; Veer, V. Development and validation of a reverse phase liquid chromatography method for the simultaneous quantification of eserine and pralidoxime chloride in drugs-in-adhesive matrix type transdermal patches. Drug Res. 2013, 63, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Seo, N.; Lee, Y.R.; Park, H.S.; Truong, Q.-K.; Lee, J.-Y.; Chung, H.K.; Choi, Y.; Kim, B.; Han, S.B.; Kim, K.H. Determination of urazamide in pharmaceutical preparation with room temperature ionic liquid. Arch. Pharmacal Res. 2017, 40, 364–372. [Google Scholar] [CrossRef] [PubMed]




| Analyte | Rt (min) | k’ | Tf | Width | Resolution | N |
|---|---|---|---|---|---|---|
| USP method a | 6.55 | 5.35 | 1.16 | 0.156 | 10.21 | 11,968 |
| KP method b | 1.12 | 0.07 | 1.69 | 0.072 | 3.17 | 1543 |
| Proposed method b | 4.05 | 2.92 | 1.68 | 0.152 | 6.14 | 5795 |
| Analyte | Column | Mobile Phase | Wavelength | Ref. |
|---|---|---|---|---|
| Pralidoxime chloride (USP) | L1 (150 mm × 4.6 mm, 3–3.5 μm) | A: 2 mM tetraethylammonium chloride and 8 mM sodium octanesulonate monohydrate in water (pH 4.3) B: Acetonitrile A/B (83:17, v/v) | 270 nm | [40] |
| Atropine, pralidoxime chloride, obidoxime chloride, and HI-6 | Agilent Zorbax Rx-C18 (250 mm × 4.6 mm, 5 μm) | A: 50 mM sodium dihydrogenorthophosphate, 1–5 mM trimethylamine, and 0.5–1 mM l-octanesulfonic acid sodium salt in water (pH 3.5) B: Acetonitrile | 203 nm | [11] |
| Pralidoxime mesylate and its degradation products | Thermo HypersilTM ODS (125 mm × 4.6 mm) | A: 0.1 M trimethylamine phosphate and 10 mM sodium lauryl sulfate in water (pH 3.0) B: Methanol A/B (90:10, v/v) | 262 nm | [13] |
| Pyridine aldoximes | Agilent Zorbax Rx-C18 (250 mm × 4.6 mm, 5 μm) | A: Sodium phosphate dibasic dehydrate, citric acid, and 1-octane sulfonic acid in water (pH 3.7) B: Acetonitrile | 276 nm | [16] |
| Pralidoxime chloride | Agilent Zorbax SB-C18 (150 mm × 4.6 mm, 5 μm) | A: 0.01% trifluoroacetic acid in water B: 0.01% trifluoroacetic acid in methanol | - (LC/MSD) | [41] |
| Eserine and pralidoxime chloride | CHOMASIL C18 (250 mm × 4.6 mm, 5 μm) | A: 10 mM potassium dihydrogen phosphate and 10 mM heptane-1-sulfonic acid in water (pH 3.0) B: Acetonitrile A/B (70:30, v/v) | 238 nm | [42] |
| Analyte | Concentration (µg/mL) | Precision (RSD, %) | Accuracy (%) | ||
|---|---|---|---|---|---|
| Intra-Day | Inter-Day | Intra-Day | Inter-Day | ||
| Pralidoxime chloride | 10 | 0.66 | 8.79 | 99.18 | 94.51 |
| 20 | 0.20 | 2.56 | 100.18 | 97.44 | |
| 30 | 0.13 | 0.65 | 100.52 | 101.01 | |
| 40 | 0.44 | 0.77 | 99.77 | 100.59 | |
| 50 | 0.47 | 0.12 | 99.96 | 100.09 | |
| Analyte | Base (µg/mL) | Spiked (µg/mL) | Found (µg/mL) | Recovery | |
|---|---|---|---|---|---|
| Mean, % | RSD, % | ||||
| Pralidoxime chloride | 26.17 | 2 | 28.04 | 99.55 | 0.36 |
| 5 | 30.88 | 99.06 | 0.53 | ||
| 10 | 35.89 | 99.22 | 0.62 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, B.; Kim, H.-s.; Lee, J.-Y.; Seo, S.; Jeong, C.H.; Bae, E.; Kim, J.; Lee, H.; Lee, D.; Lee, D.-K.; et al. Unveiling Novel Chaotropic Chromatography Method for Determination of Pralidoxime in Nerve Agent Antidote Autoinjectors. Separations 2024, 11, 82. https://doi.org/10.3390/separations11030082
Shin B, Kim H-s, Lee J-Y, Seo S, Jeong CH, Bae E, Kim J, Lee H, Lee D, Lee D-K, et al. Unveiling Novel Chaotropic Chromatography Method for Determination of Pralidoxime in Nerve Agent Antidote Autoinjectors. Separations. 2024; 11(3):82. https://doi.org/10.3390/separations11030082
Chicago/Turabian StyleShin, Bohyun, Hyung-seung Kim, Ji-Youn Lee, Sumin Seo, Cho Hee Jeong, Eunbin Bae, Jiyu Kim, Hyojeong Lee, Donghee Lee, Dong-Kyu Lee, and et al. 2024. "Unveiling Novel Chaotropic Chromatography Method for Determination of Pralidoxime in Nerve Agent Antidote Autoinjectors" Separations 11, no. 3: 82. https://doi.org/10.3390/separations11030082
APA StyleShin, B., Kim, H. -s., Lee, J. -Y., Seo, S., Jeong, C. H., Bae, E., Kim, J., Lee, H., Lee, D., Lee, D. -K., & Han, S. B. (2024). Unveiling Novel Chaotropic Chromatography Method for Determination of Pralidoxime in Nerve Agent Antidote Autoinjectors. Separations, 11(3), 82. https://doi.org/10.3390/separations11030082