1. Introduction
Canines are a vital biological detector tool for various law enforcement agencies, with different detection applications including explosives, narcotics, human remains, and human scent [
1]. Human scent itself can be described as a mixture of numerous volatile organic compounds (VOCs) released from the skin, breath, and bodily fluids, resulting in a highly varied human biochemistry that can be analyzed via noninvasive approaches with appropriate headspace instrumental techniques [
2,
3,
4]. As a form of trace evidence, human scent can aid in criminal investigations by determining the travel direction of a suspect, identifying the suspect through a scent line-up procedure, or aiding in victim search and recovery efforts [
3]. This type of evidence is commonly paired with human scent detection canines, because these biological detectors are specifically trained to identify and locate the scent of live humans [
5]. Although a plethora of research within human scent detection disciplines already exists, there is a current gap in understanding the odor picture of the constituents of the human body that are of importance for canine response and perception. Therefore, it is important to understand the volatile odor chemical characterization of a whole human and how it may differ from the chemical odor profile of individual biospecimens (hand, axillary, breath), to understand and better inform sensor development and canine human scent detection. In 2014, a study reported a total of 1846 compounds across different biospecimens from healthy individuals to include a range of chemical classes, such as aldehydes, short- and long-chain hydrocarbons, carboxylic acids, alcohols, esters, ketones, and amines [
6]. Further work in the human volatilome continued with over 900 new compounds being reported in an updated compendium in 2021 [
7]. While the study of human scent has increased over the years, it is important to understand the complexity of this type of analysis. Many variables come into play, including instrumental headspace analysis techniques, methods and body area of collection, subject characteristics (gender, lifestyle, medical history), and environment. Hence, human scent odor signatures are complex and dynamic, posing a challenge for chemical and biological detection.
Chemical odor characterization in support of canine detection has been performed on a wide variety of target odors, with human scent being one of the main sources of study. Human scent is a widely utilized canine detection discipline within law enforcement and search and rescue operations [
8]. Human scent detection canines can be used to establish an association between human scent traces left at a given location or in pre-scenting scenarios in which the canine is given a scented article to follow an associated scent trail to a specific person or location. Within search and rescue applications, a more generalized definition of human scent is the target for detection, as the canine’s goal is to locate live human victims in disaster events for victim recovery efforts. Gunter et. al., for example, took the approach of general body odor sampling through sensor arrays to better understand the “entrapped” human odor signature for natural disaster situations [
9].
Human scent detection can be broadly categorized into (1) generalization of a live human scent odor signature and (2) individualization of a specific subject in a discrimination scenario. Regardless of the application, a central gap in knowledge with respect to human scent detection is the identification of the human volatile markers perceived by dogs to make the identification or, even yet, the most productive compounds for sensor development in cases of technology advancements aiming to help first responders locate victims in disaster environments [
9].
Volatolomics is a current area of much research in many different applications to understand the composition, origin, and detection of volatile biomarkers from the human body. These areas have included biomedical research (disease diagnostic tools, cosmetic/hygiene industry) [
10,
11,
12], textile odor control technologies [
13], and forensic/security applications (human biometric potential, search and rescue, human smuggling) [
9,
14,
15]. Volatile organic compounds having high vapor pressures resulting from exogenic and endogenic sources together form an area of study known as the human “volatilome” [
16].
Within an analytical perspective, the challenge for human odor volatile analysis remains in the array of collection techniques, sample types, and detection approaches utilized to perform these studies [
2]. While previous research has delved into many different types of human biospecimens for analysis [
2,
7], an area that has yet to be fully understood is the analysis of the whole human body as the source for sampling purposes. More so is understanding the human body as an emission odor source and how this full human sample relates to its sublayers, as seen in the many biospecimens that make the whole-body function from a physiological, metabolic, genetic, and bacterial perspective. To this end, few studies have embarked on utilizing chamber-like approaches to capture the human odorprint for analysis [
9,
17,
18,
19]. Nonetheless these studies have not evaluated how the whole-body odor picture differs from the odor picture generated by individual body parts.
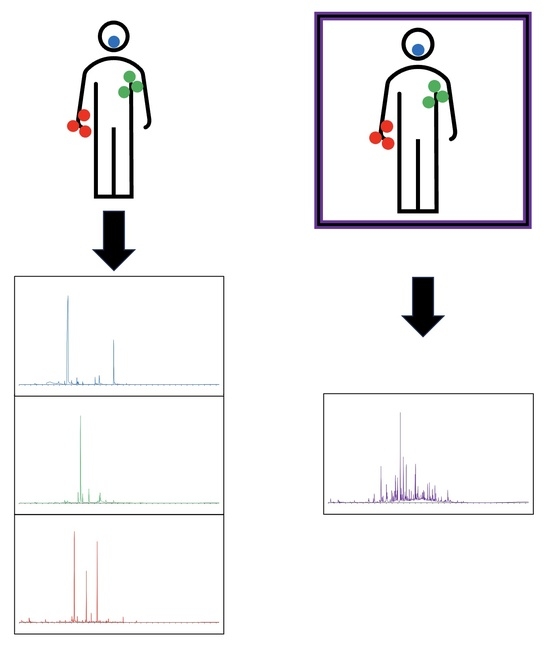
To continue in this effort, the objective of this experiment was to provide a laboratory-controlled whole-body odor headspace sampling approach from a cohort of ten human subjects (five females and five males). Furthermore, we performed parallel collection of additional biospecimens to include hand, axillary, and exhaled breath from the same individuals to provide a comparative approach of each sampling technique. For this effort, we developed a chamber for human whole-body odor samplings that allowed for a human participant to be sampled in a chamber-like approach using SPME-GC/MS as the technique for headspace vapor sampling.
Previous research by the authors used this approach for canine behavioral assessments to better understand key human biomarkers for scent detection purposes [
20].. The results of this study depicted that from a detection canine’s perspective, human scent consists of volatiles from various origins, with exhaled breath volatiles being an important component [
20]. Thus, chemical validation of the chamber can assist in identifying what is the main constituent of human scent from the perspective of a canine [
20]. Pairing these results with those of the present study can aid in bridging a gap between canine behavior and chemical component characterization of human scent.
2. Materials and Methods
2.1. Whole-Body Human Sampling
The chamber was made of a clear acrylic box (81.21 × 81.28 × 91.44 cm). The top side of the box was removable to allow access to the interior. The box was sealed with acrylic cement (Craftics
® #33; Los Angeles, CA USA) and a rubber draft seal strip (Cloud Buyer Professional sealing solution) to reduce extraneous air intrusion. An air pump provided the chamber with clean filtered air (~10 L/m) via a nasal canula. This provided volunteers a constant surplus of fresh air. Four holes were drilled into the walls of the chamber to insert SPME fibers for human sampling. These holes were located 2 ¼ in from each side and 12 inches from the ground. They would serve as a type of injector port for the SPME fibers so that they could easily be injected by the experimenter from the outside of the chamber to prevent any disturbance or contamination. The corners were each given an identifiable number (Location 1–4), as can be seen in
Figure 1.
Prior to human sampling, the chamber was cleaned with a 50:50 dilution of isopropyl alcohol and deionized (DI) water. The inside walls, as well as the floor and top of the chamber, were cleaned with the alcohol solution and allowed to air dry for 30 min with the lid off. The steps were repeated a second time. After the second air dry cycle, the lid was placed on the chamber, and a 1 h blank extraction was initiated. This blank would serve as a baseline analysis of the empty box, and any compounds present in the empty box would be subtracted from the human sample.
Participants were first provided with Natural, Clear Olive Oil Soap from Life of the Party and instructed to wash with it at least once within the 24 h period prior to sampling. This procedure was similar to that described in a study performed by Curran et al. [
21]. However, in the present study, a slight modification was made, as the timeline of washing with the soap was changed from 48 h to 24 h prior to sampling. This research was approved by the Human Research Protection Program, Office of the Vice President for Research at Texas Tech University under IRB protocol number IRB2022-236.
All subjects were sampled in indoor laboratory conditions with the pretreated cotton gauze pads. No dietary restrictions were placed on tested participants prior to any hand, axillary, or breath odor collection. However, for axillary odor sampling, participants were directed to shower with the provided olive oil soap at least once during the 24 h period prior to sampling (chamber and biospecimen). Additionally, participants were instructed to discontinue any use of deodorants, lotions, and perfumes after washing with the soap. Whole-body sampling via the chamber took place one day, and individual biospecimen collection occurred within a week of chamber sampling due to subject sampling time constraints. All biospecimens (hand, axillary, and breath) were collected on the same day.
On the day of sampling, participants entered the chamber through the top. Experimenters would then close the chamber by replacing the lid, thus building the headspace for sampling. The chamber was not locked, allowing volunteers to open the chamber at any time during testing, if need be. Once the participant was inside, the SPME fibers were placed in their respective locations and injected for a 1 h extraction period. Once the extraction period was finished, the SPME fibers were retracted and removed. Experimenters would then remove the lid of the chamber to allow for the participant to exit.
2.2. Pre-Treatment of Collection Materials
The collection materials for biospecimens consisted of cotton gauze pads. The gauze pads were 100% cotton, sterile, 2 in. × 2 in., 8-ply gauze sponges (Dukal Corporation, Syosset, NY, USA). The vials used for sample extraction and storage were 15 mL clear glass screw-top vials with PTFE/Silicone septa (Supelco, Bellafonte, PA, USA). BACtrack Professional Breathalyzer Mouthpieces were utilized for breath collection (BACKtrack, San Francisco, CA, USA). While these materials are biologically sterile, this does not equate to an analytically clean collection material [
22]. In order to remove any remnants of intrinsic textile volatile organic compounds (VOCs) that could lead to background contamination, all materials used for human sampling underwent a pretreatment prior to use following previous procedures from the authors [
22], which involved spiking the materials with HPLC-grade methanol, then heating them in the oven at 105 °C. Vials were heated for 2 h, while their lids and septa were heated for 15 min. For gauze pretreatment, small circular stands were created from floral wire and were then placed inside a clear Pyrex baking pan. This set-up made it possible for heat to penetrate the gauze from all directions and remove volatile remnants. The stands and Pyrex pan were first spiked with methanol and then heated in the oven for 30 min. After preliminary heating, gauze pads were placed on top of each stand, saturated with methanol, and allowed to bake for four hours. Each collection material was analyzed by SPME-GC⁄MS (same method as that used for scent samples later described in the text) for compound identification and verification of blank background prior to sampling use. After sterilization, the pre-treated gauze was stored in the sterile 15 mL vials. Mouthpieces were placed in a beaker, saturated with methanol, and allowed to bake for 15 min.
2.3. Hand Odor Sampling
Triplicate samples of each subject were taken sequentially on the same day. To collect the hand odor from each subject, a contact collection process was followed from previous work by the authors [
22]. This contact collection process entails an initial washing of the hands for 30 s using Natural, Clear Olive Oil Soap from Life of the Party. The preliminary wash was then followed by a 2 min rinse under warm water and then a 1 min air dry. The participants were then asked to rub their forearms and palms together for 2 min. Once this procedure was completed, they were given a pre-treated gauze pad and were asked to hold it in between their palms for 10 min. Subjects were allowed a 5 min break in between samples. The collected samples were then placed back into their respective 15 mL glass vials at room temperature and allowed to equilibrate for 24 h prior to extraction. A 21 h headspace SPME extraction followed the allotted headspace equilibration.
2.4. Axillary Odor Sampling
Duplicate (one per armpit) samples of each subject were taken sequentially on the same day. To collect the axillary odor from each subject, a contact collection process was followed from previous work by the authors [
21]. The subject was first taken on a 10 min walk outside, where the temperature and humidity were recorded. After the 10 min, the subject was brought back into the lab, where they were given the pre-treated gauze and instructed to place one pad under their right axillary area and one pad under their left axillary area. The gauze remained there for 5 min before it was collected from each axillary area and placed into the 15 mL glass vials at room temperature and allowed to equilibrate for 24 h prior to extraction. A 21 h headspace SPME extraction followed the allotted headspace equilibration.
2.5. Breath Odor Sampling
Duplicate samples of each subject were taken sequentially on the same day. To collect the breath odor from each subject, they were first given a Dixie™ cup to rinse their mouth with regular water for ten (10) seconds. After this rinse, subjects were given one pre-treated mouthpiece and two 15 mL vials—each containing pre-treated gauze. Subjects were instructed to inhale through their nose, remove the lid of the vial, place the mouthpiece into the opening of the vial, and then blow into the vial through the mouthpiece for ten (10) seconds. Subjects were then instructed to remove the mouthpiece and replace the lid on the vial, before repeating these steps with their second vial. The samples were stored at room temperature and allowed to equilibrate for 24 h prior to extraction.
2.6. Participants
A total of ten (10) participants were used for this experiment. To provide a representative sampling of both genders, 5 males and 5 females were evaluated. Previous studies have shown that there are gender differences with respect to human scent composition. Hand odor VOC marker combinations have been used to classify individuals by their gender [
23] as well as gender differences within the axillary microbiome [
24]. Thus, the experiment incorporated an equal number of each gender to provide an equitable gender sample framework. The proposed target population included students, staff, or faculty from the department, or anyone 18 years or older, as the target population did not require specific characteristics. The average age for all participants was 25.5 years of age. With respect to male subjects, the average age was 25 years, with the youngest being 19 and the oldest being 37. As for the female participants, the average age was 26 years, with the youngest being 19 and the oldest being 42.
2.7. Solid-Phase Microextraction (SPME)
Headspace extractions of all collected samples utilized divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fibers with an exposure time of 21 h at room temperature [
22] for biospecimens and a 1 h exposure time for chamber samples. This specific fiber type was chosen in accordance with the optimal fiber for the extraction of human scent samples [
21]. Prior to any sampling, each SPME fiber was conditioned for three 30 min sessions at an oven temperature of 250 °C to ensure that the fibers were clean and ready for use. Following this pretreatment, a blank fiber instrument run was performed to guarantee an absence of contaminants or lingering volatiles on the fiber.
SPME is a sample preparation tool that is both versatile and non-exhaustive and has proven itself to be well-suited for easy and effective analysis of different compounds in various studies. This technique consists of two basic steps: the partitioning, or separating, of analytes between the extraction phase and the sample matrix and the desorption of concentration extracts into an analytical instrument.
2.8. Gas Chromatography Mass Spectrometry (GC/MS) Method
An Agilent Technologies GC 7890A with an Agilent Technologies 5975C inert XL MSD with triple-axis detector (Agilent Technologies, Santa Clara, CA, USA) was the instrument used to separate and analyze compounds. A Rtx®-5 capillary 30 m × 250 µm × 0.25 µm column (Restek Corporation, Bellefonte, PA, USA) was used. Helium was the carrier gas with a flow rate of 1.0 mL/min. The GC oven temperature ramp was programmed from 40 °C to 300 °C beginning with a 5 min hold at 40 °C. The GC then heated up at a rate of 10 °C/min up to 300 °C and held for 2 min, for a total run time of 33 min. The analysis was conducted under splitless mode. The inlet had an initial temperature of 250 °C with a pressure of 7.1 psi. The total flow was 18.003 mL/min. The mass spectrometer was operated in electron ionization mode and scanned over a mass range of m/z 45–550 in full scan mode.
Considering that there was a plethora of compounds seen in this study, several compounds that were detected across all sampling types were chosen to create liquid solutions of various concentrations (5–100 ppm) to create external calibration curves for quantitation purposes. This was carried out by injecting the liquid solutions into the GC/MS system using the same method used for headspace sampling. An average response factor was then obtained and used to approximate the amount of VOCs being extracted by the SPME, considering that the slope of the calibration line represents the response for each compound analyzed.
Table 1 depicts the limits of detection (LOD) and limits of quantitation (LOQ) for the selected compounds for the calibration curves.
2.9. Data Analysis
Compounds were identified using the NIST 17 (2017) mass spectral reference library. The criteria for the compounds identified were those with detected peaks greater than or equal to a mass spectral quality of 80% or above. For the whole-body odor samplings (chamber), primary odor volatiles were defined as compounds present in all four sampling locations. For biospecimen samplings, primary odor compounds were defined as those present in all triplicate samples (hand odor samples) and all duplicate samples (axillary odor and breath samples). Duplicate sample collection was performed on axillary and breath samples due to restrictions of a subject having only two axillary areas and to further reduce subject sampling time commitment while providing replicates for each specimen. Volatiles detected from blank chamber samplings were deducted from human sampling when needed to provide for a corrected background subtraction. When referring to high-frequency occurring compounds, these are the compounds present in at least 50% (5 out of 10) of the subjects in order to produce a snapshot of whole-body odor. All generated data were analyzed via Chemstation software, version 10.1.49 (Agilent Technologies, Santa Clara, CA, USA) and the National Institute of Standards and Technology mass spectral library (NIST 2017) for compound identification. In order to monitor the clustering of the obtained chemical odor profile, principal component analysis (PCA) was used to reduce the amount of data using the data’s correlation matrix. Using the correlation matrix allowed the data to be standardized, having each variable equal to zero mean and unit variance. As the variables in this case were the detected compounds for each subject, standardization allowed for the variables to be measured with equal weights. Statistical analysis was performed using JMP Pro 16.0.0, SAS Institute Inc., Cary, NC, USA, 2021.
4. Discussion
While whole-human body odor analytical perspectives are not novel, this study corroborates previous studies [
17,
18] utilizing chamber collection approaches depicting both nonanal and decanal as key odor markers. In addition, Rankin-Turner and McMeniman reported seeing an array of ketones, aldehydes, hydrocarbons, and alcohols, which were also seen in the present study [
18]. Ketones, such as 6-Methyl-5-hepten-2-one and 6,10-Dimethyl-5,9-undecadien-2-one; hydrocarbons like hexadecane and tetradecane; and other aldehydes like benzaldehyde and heptanal (in addition to nonanal and decanal) were all shared compounds between the present study and that performed by Rankin-Turner and McMeniman [
18]. In contrast to this reported study, our collection approach did not yield a high abundance of acids. Similar to the present study, Zou et al. also reported a lack of acid detection among their whole-body sampling [
17]. This was deemed to be a limitation of their collection substrate (Tenax-TA tubes) as well as the chromatographic columns used for their analysis [
17]. Additionally, Zou et al. [
17] focused on VOCs strictly emitted from whole-body skin, and, considering that the skin was the most highly exposed via the chamber odor collection in the present study, these results corroborate those seen in the previous literature. These compound classes have been previously reported as originating from metabolism of skin lipids and bacterial degradation [
41]. Given that carboxylic acids tend to be heavier than other groups, this could explain their lack of presence in the current study. The sampling technique via the chamber could have potentially created a headspace too large for the acids to reach the SPME fiber, and thus, only compounds with higher volatility were able to reach the SPME fiber. It is important to note that human scent sampling techniques have intrinsic limitations to the results obtained and, hence, make the reproducibility across studies challenging for result merging. In this particular study, the DVB/CAR/PDMS SPME fiber was utilized for headspace odor sampling. This fiber was selected by the authors in previous studies as optimal for odor collection. However, the large headspace area surrounding the human subject within the chamber yields to variations in the amounts of compounds absorbed by the SPME sampling devices. Future studies should investigate alternate fiber chemistries within this chamber context as well as evaluate higher fiber numbers during sampling to optimize potential vapor sampling limitations of the SPME technique.
When comparing the whole body and individual biospecimens, the surface area and body sampling regions can lead to varying VOCs, as different locations have different microbial populations, and thus, different biotransformation processes that result in the release of VOCs [
42]. The chamber sampling method allowed for the entire human body to be exposed, thus providing a higher surface area of the human body available for VOC collection. With this enhanced “body exposure availability,” a wider range of VOCs could potentially be in the available headspace and available for detection. This can lend a hand in explaining why some compounds were detected via chamber sampling versus individual biospecimens. When the sampling location is reduced to a smaller body part, such as the palms of the hands—which consist mainly of eccrine sweat glands [
42]—the biotransformation processes may differ and thus lead to a slightly different odor profile.
The constituents of an individual that remain constant across time regardless of diet/environmental factors are termed the primary odor, while the secondary odor is composed of those constituents that are a result of the diet/environmental factors. Lastly, constituents that exist due to outside sources, such as lotions, soaps, etc., are described as the tertiary odor [
21]. Therefore, the variation in primary compounds observed between individuals and sample types could be a result of any of these aforementioned factors. In the present study, primary compounds were those recurring compounds in the replicate samples of the same individual for the particular sample type. For example, compounds present in all three hand samples of one individual were primary compounds of their hand odor, while compounds present in their two breath samples were primary compounds of the breath odor. Based on the results obtained, an intrinsic intra-subject variation across samples is observed, therefore representing the complexity of the human scent profile across replicate samples of a single subject. This reinforces the importance of obtaining more than one sample from a single person, as one sample is not enough to provide a reproducible snapshot of an odor profile; thus, multiple samples are needed to create a baseline for a single subject when conducting these types of analyses. It is also important to note that other studies comparing human odor volatiles across distinctive biological specimens have also noted inter-specimen VOC differentiation from the same individual [
3,
27]. These studies have depicted the applicability of emanating volatile organic compounds from various forensic specimens for individual differentiation, thus reinforcing the notion of the complexity of the human volatilome.
As previously mentioned, the idea of sampling human scent using a chamber-like device is not novel, as previous studies have already used this approach to analyze breath- and skin-emitted metabolic tracers, as well as the whole-body volatilome in general [
9,
17,
18]. However, the present study is novel, as it can bridge the gap between understanding the chemical characterization of whole-body human scent and individual biospecimens of the same population being sampled. This study embarks on an analysis of sampling the same subject in different contexts to capture the human scent landscape from multiple lenses. Aside from the human sampling context, the chamber developed represents a path forward for canine detection applications. Previous studies by the authors have utilized the same chamber, coupled with an olfactometer system, to expose canines to human scent in a controlled setting and evaluate their response to various scented articles and individual components of human scent [
20]. It was found that the chamber had the capability of capturing human scent and when paired with the olfactometer system, had the ability to present the headspace of the chamber to canines in a separate room. Additionally, the canines were able to discriminate the chamber containing a human subject from chambers containing distractor odors [
20]. These results, along with those procured from the present study, validate that the chamber is able to capture VOCs emanating from a whole human body and that these VOCs are a good representation of human scent for search and rescue (SAR) dogs [
20].
5. Conclusions
This study aimed to develop a whole-body odor sampling approach to test the capability of a human chamber to yield detectable odor signature via instrumental means. The chamber was used in a laboratory setting to obtain the odor profile from a set of ten (10) participants. Human headspace samples were collected via four strategically placed SPME fibers within the cavity of the chamber to extract participant odor VOCs. This instrumental capability allowed the chemical characterization of a range of previously reported human odor volatiles, including frequent classes such as aldehydes and aliphatics. Whole-human body odor depicted the highest number of reported VOCs, thereby showcasing a distinction from individual human biospecimens such as hand, axillary, and exhaled breath samples. The chamber whole-human body odor samples depicted statistical clustering as a function of gender, reinforcing the published literature of the capability of VOC odor profiles to categorize individuals by gender. Future studies can exploit this body sampling approach to integrate with canine testing or to extend its applicability in volatilomic work within medical diagnostic and forensic applications to further understand the complex human scent signature.
While nonanal and decanal were the only compounds detected across all four sample types, this study corroborates previous studies within the human scent discipline, as commonly reported compounds were identified. While this study only focused on the analysis of ten (10) individuals, future studies could expand the number of participants. This expansion in the number of participants will also add to the pre-existing list of human scent compounds that have been reported thus far. While personal hygiene restrictions were placed on the subjects of this study, dietary restrictions were not. This could account for certain compounds seen in the results. Future studies that seek to collect human VOCs could place dietary restrictions or stricter personal hygiene restrictions on their participants to control and monitor extraneous odor contaminations.
Overall, the present study showed that human body sampling is feasible via SPME-GC/MS, as a total of 105 compounds were identified from the ten (10) subject participants tested. Additionally, the methods used allowed for the collection of hand, axillary, and breath odor volatiles to provide a biospecimen comparison to the chamber sampling approach. Whole-body odor volatiles were successfully sampled, allowing for this chamber system not only to allow for chemical characterization but also to be concurrently utilized in an olfactometer set-up for canine odor perception studies of human scent. While the breath collection method yielded only ten compounds, the compounds were commonly reported breath VOCs. Statistical analysis showed that the odor profiles collected from the whole-human body sampling clustered closer to each other, while the odor profiles of the individual biospecimens clustered amongst each other. This information can be valuable for future studies that wish to identify the prominent compounds within human scent detected by canines and provide a framework of study for human scent odor signatures.