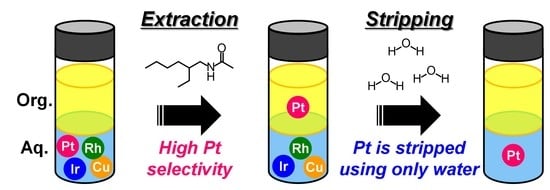
Selective Extraction of Platinum(IV) from the Simulated Secondary Resources Using Simple Secondary Amide and Urea Extractants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of N-(2-Ethylhexyl)acetamide (MonoAA)
2.3. Synthesis of N-(2-Ethylhexyl)octanamide (MonoOA)
2.4. Synthesis of N,N-Bis(2-ethylhexyl)acetamide (BisAA)
2.5. Synthesis of 1-Butyl-3-(2-ethylhexyl)urea (MonoBU)
2.6. Metal Ion Extraction Procedure
3. Results and Discussion
3.1. Extraction from Lower Metal Concentration
Effect of HCl Concentration
3.2. Extraction from SSRs
3.2.1. Effect of Shaking Time
3.2.2. Pt(IV) Separation from SSRs
3.2.3. Effect of Diluent Composition of the Organic Phase
3.2.4. Effect of Phase Ratio of the Organic and Aqueous Phases
3.2.5. Effect of Pt(IV) Concentration
3.3. Stripping Test
3.3.1. Stripping of Pt(IV)
3.3.2. Effect of Settling Time of Organic Phase after Pt(IV) Loading
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, J.; Beecham, J. A Study of Platinum Group Metals in Three–Way Autocatalysts. Platin. Met. Rev. 2013, 57, 281–288. [Google Scholar] [CrossRef]
- Hagelüken, C. Recycling the Platinum Group Metals: A European Perspective. Platin. Met. Rev. 2012, 56, 29–35. [Google Scholar] [CrossRef]
- Glaister, B.J.; Mudd, G.M. The Environmental Costs of Platinum–PGM Mining and Sustainability: Is the Glass Half-Full or Half-Empty? Miner. Eng. 2010, 23, 438–450. [Google Scholar] [CrossRef]
- Holton, O.T.; Stevenson, J.W. The role of platinum in proton exchange membrane fuel cells. Platin. Met. Rev. 2013, 57, 259–271. [Google Scholar] [CrossRef]
- Bernardis, F.L.; Grant, R.A.; Sherrington, D.C. A Review of Methods of Separation of the Platinum-Group Metals through Their Chloro-Complexes. React. Funct. Polym. 2005, 65, 205–217. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2020: U.S. Geological Survey; U.S. Geological Survey: Reston, VA, USA, 2020; ISBN 978-1-4113-4362-7.
- Narita, H.; Kasuya, R.; Suzuki, T.; Motokawa, R.; Tanaka, M. Precious Metal Separations. Ency. Inorg. Bioinorg. Chem. 2020, eibc2756, 1–28. [Google Scholar] [CrossRef]
- Jha, M.K.; Gupta, D.; Lee, J.-C.; Kumar, V.; Jeong, J. Solvent Extraction of Platinum using Amine Based Extractants in Different Solutions: A review. Hydrometallurgy 2014, 142, 60–69. [Google Scholar] [CrossRef]
- Levitin, G.; Schmuckler, G. Solvent Extraction of Rhodium Chloride from Aqueous Solutions and Its Separation from Palladium and Platinum. React. Funct. Polym. 2003, 54, 149–154. [Google Scholar] [CrossRef]
- Costa, M.C.; Almeida, R.; Assunção, A.; Costa, A.M.R.; Nogueira, C.; Paiva, A.P. N,N′-tetrasubstituted succinamides as new molecules for liquid–liquid extraction of Pt(IV) from chloride media. Sep. Purif. Technol. 2016, 158, 409–416. [Google Scholar] [CrossRef]
- Paiva, A.P.; Carvalho, G.I.; Costa, M.C.; Costa, A.M.R.; Nogueira, C. The solvent extraction performance of N,N′-dimethyl-N,N′-dibutylmalonamide towards platinum and palladium in chloride media. Sep. Sci. Technol. 2014, 49, 966–973. [Google Scholar] [CrossRef]
- Costa, M.C.; Assunção, A.; Costa, A.M.R.; Nogueira, C.; Paiva, A.P. Liquid-Liquid Extraction of Platinum from Chloride Media by N,N′-Dimethyl-N,N′- Dicyclohexyltetradecylmalonamide. Solvent Extr. Ion Exch. 2013, 31, 12–23. [Google Scholar] [CrossRef]
- Maeda, M.; Narita, H.; Tokoro, C.; Tanaka, M.; Motokawa, R.; Shiwaku, H.; Yaita, T. Selective Extraction of Pt(IV) over Fe(III) from HCl with an Amide-Containing Tertiary Amine Compound. Sep. Purif. Technol. 2017, 177, 176–181. [Google Scholar] [CrossRef]
- Warr, R.J.; Bell, K.J.; Gadzhieva, A.; Cabot, R.; Ellis, R.J.; Chartres, J.; Henderson, D.K.; Lykourina, E.; Wilson, A.M.; Love, J.B.; et al. A Comparison of the Selectivity of Extraction of [PtCl6]2− by Mono-, Bi-, and Tripodal Receptors That Address Its Outer Coordination Sphere. Inorg. Chem. 2016, 55, 6247–6260. [Google Scholar] [CrossRef]
- Carson, I.; MacRuary, K.J.; Doidge, E.D.; Ellis, R.J.; Grant, R.A.; Gordon, R.J.; Love, J.B.; Morrison, C.A.; Nichol, G.S.; Tasker, P.A.; et al. Anion Receptor Design: Exploiting Outer-Sphere Coordination Chemistry To Obtain High Selectivity for Chloridometalates over Chloride. Inorg. Chem. 2015, 54, 8685–8692. [Google Scholar] [CrossRef]
- Ellis, R.J.; Chartres, J.; Henderson, D.K.; Cabot, R.; Richardson, P.R.; White, F.J.; Schröder, M.; Turkington, J.R.; Tasker, P.A.; Sole, K.C. Design and Function of Pre-Organised Outer-Sphere Amidopyridyl Extractants for Zinc(II) and Cobalt(II) Chlorometallates: The Role of C–H Hydrogen Bonds. Chem. Eur. J. 2012, 18, 7715–7728. [Google Scholar] [CrossRef]
- Warr, R.J.; Westra, A.N.; Bell, K.J.; Chartres, J.; Ellis, R.; Tong, C.; Simmance, T.G.; Gadzhieva, A.; Blake, A.J.; Tasker, P.A.; et al. Selective extraction and transport of the [PtCl6]2− anion through outer-sphere coordination chemistry. Chem. Eur. J. 2009, 15, 4836–4850. [Google Scholar] [CrossRef]
- Bell, K.J.; Westra, A.N.; Warr, R.J.; Chartres, J.; Ellis, R.; Tong, C.C.; Blake, A.J.; Tasker, P.A.; Schröder, M. Outer-Sphere Coordination Chemistry: Selective Extraction and Transport of the [PtCl6]2− Anion. Angew. Chem. Int. Ed. 2008, 47, 1745–1748. [Google Scholar] [CrossRef]
- Swiegers, G.F.; Malefetse, T.J. Multiple-Interaction Self-Assembly in Coordination Chemistry. J. Incl. Phenom. Macrocycl. Chem. 2001, 40, 253–264. [Google Scholar] [CrossRef]
- Philp, D.; Stoddart, J.F. Self-Assembly in Natural and Unnatural Systems. Angew. Chem. Int. Ed. 1996, 35, 1154–1196. [Google Scholar] [CrossRef]
- Ohto, K.; Ueda, Y.; Ohmi, K.; Fujita, M. Platinum extractant, platinum extraction method, and platinum recovery method, JP Patent. JP6556685B2, 7 August 2019. [Google Scholar]
- Narita, H.; Morisaku, K.; Tanaka, M. The first effective extractant for trivalent rhodium in hydrochloric acid solution. Chem. Commun. 2008, 5921–5923. [Google Scholar] [CrossRef]
- Low, C.M.R.; Broughton, H.B.; Kalindjian, S.B.; McDonald, I.M. Novel oxathiazinones as gastrin ligands: Unezpected products from the Schotten-Baumann reaction of arylsulphonyl chlorides with derivatives of aspartic acid. Bioorg. Med. Chem. Lett. 1992, 2, 325–330. [Google Scholar] [CrossRef]
- Erne, D.; Stojanac, N.; Ammann, D.; Hofstetter, P.; Pretsch, E.; Simon, W. Lipophilic Di- and Triamides as Ionophores for Alkaline Earth Metal Cations. Helv. Chim. Acta 1980, 63, 2271–2279. [Google Scholar] [CrossRef]
- Sonntag, N.O.V. The Reactions of Aliphatic Acid Chlorides. Chem. Rev. 1953, 52, 237–416. [Google Scholar] [CrossRef]
- Raposo, C.; Almaraz, M.; Martin, M.; Weinrich, V.; Mussóns, L.; Alcázar, V.; Caballero, C.; Morán, J.R. Tris(2-aminoethyl)amine, A Suitable Spacer for Phosphate and Sulfate Receptors. Chem. Lett. 1995, 24, 759–760. [Google Scholar] [CrossRef]
- Amendola, V.; Fabbrizzi, L.; Mosca, L. Anion Recognition by Hydrogen Bonding: Urea-Based Receptors. Chem. Soc. Rev. 2010, 39, 3889–3915. [Google Scholar] [CrossRef]
- Hay, B.P.; Firman, T.K.; Moyer, B.A. Structural Design Criteria for Anion Hosts: Strategies for Achieving Anion Shape Recognition through the Complementary Placement of Urea Donor Groups. J. Am. Chem. Soc. 2005, 127, 1810–1819. [Google Scholar] [CrossRef]
- Koevoets, R.A.; Versteegen, R.M.; Kooijman, H.; Spek, A.L.; Sijbesma, R.P.; Meijer, E.W. Molecular Recognition in a Thermoplastic Elastomer. J. Am. Chem. Soc. 2005, 127, 2999–3003. [Google Scholar] [CrossRef] [PubMed]
- Corbin, P.S.; Zimmerman, S.C.; Thiessen, P.A.; Hawryluk, N.A.; Murray, T.J. Complexation-Induced Unfolding of Heterocyclic Ureas. Simple Foldamers Equilibrate with Multiply Hydrogen-Bonded Sheetlike Structures. J. Am. Chem. Soc. 2001, 123, 10475–10488. [Google Scholar] [CrossRef]
- Etter, M.C.; Lipkowska, Z.U.; Ebrahimi, M.Z.; Panunto, T.W. Hydrogen Bond Directed Cocrystallization and Molecular Recognition Properties of Diarylureas. J. Am. Chem. Soc. 1990, 112, 8415–8426. [Google Scholar] [CrossRef]
- Pang, G.; Morisada, S.; Kawakita, H.; Hanamoto, T.; Umecky, T.; Ohto, K.; Song, X.-M. Allosteric extraction of a second gallium anion assisted by the first, loaded onto a fluorinated secondary amide reagent. Sep. Purif. Technol. 2021, 119036, In Press. [Google Scholar] [CrossRef]
- Ueda, Y.; Morisada, S.; Kawakita, H.; Ohto, K. High extraction ability and selectivity of a tripodal pivalamide derivative for Pt(IV) from hydrochloric acid solutions. Sep. Sci. Technol. 2016, 51, 2700–2707. [Google Scholar] [CrossRef]
- Ueda, Y.; Morisada, S.; Kawakita, H.; Wenzel, M.; Weigand, J.J.; Ohto, K. Effective extraction of Pt(IV) as [PtCl6]2− from hydrochloric acid using a simple urea extractant. Sep. Purif. Technol. 2021, 277, 119456. [Google Scholar] [CrossRef]
- Cox, M. Solvent Extraction in Hydrometallurgy. In Solvent Extraction Principles and Practice, 2nd ed.; Rydberg, J., Cox, M., Musikas, C., Choppin, G.R., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 455–505. [Google Scholar]
- Clement, O.; Rapko, B.M.; Hay, B.P. Structural Aspects of Metal–Amide Complexes. Coord. Chem. Rev. 1998, 170, 203–243. [Google Scholar] [CrossRef]
- Valentine, B.; Amour, T.E.S.; Fiat, D. A 17O NMR Study of the Protonation of Urea. Org. Magn. Reson. 1984, 22, 697–700. [Google Scholar] [CrossRef]
- Doidge, E.D.; Carson, I.; Tasker, P.A.; Ellis, R.J.; Morrison, C.A.; Love, J.B. A Simple Primary Amide for the Selective Recovery of Gold from Secondary Resources. Angew. Chem. Int. Ed. 2016, 26, 12436–12439. [Google Scholar] [CrossRef]
- Ortet, O.; Santos, M.S.C.S.; Paiva, A.P. Palladium(II) and N,N′-dimethyl-N,N′-dicyclohexylthiodiglycolamide—The extracted species from concentrated chloride solutions. Sep. Purif. Technol. 2016, 170, 1–9. [Google Scholar] [CrossRef]
- Turkington, J.R.; Bailey, P.J.; Love, J.B.; Wilson, A.M.; Tasker, P.A. Exploiting Outer–Sphere Interactions to Enhance Metal Recovery by Solvent Extraction. Chem. Commun. 2013, 49, 1891–1899. [Google Scholar] [CrossRef]
- Muñoz-Páez, A.; Pappalardo, R.R.; Marcos, E.S. Determination of the Second Hydration Shell of Cr3+ and Zn2+ in Aqueous Solutions by Extended X-ray Absorption Fine Structure. J. Am. Chem. Soc. 1995, 117, 11710–11720. [Google Scholar] [CrossRef]
- Choppin, G.R. Complexation of Metal Ions. In Solvent Extraction Principles and Practice, 2nd ed.; Rydberg, J., Cox, M., Musikas, C., Choppin, G.R., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 81–107. [Google Scholar]
- Roberts, J.E.; Schnitler, J. Ionic Quadrupolar Relaxation in Aqueous Solution: Dynamics of the Hydration Sphere. J. Phys. Chem. 1993, 97, 5410–5417. [Google Scholar] [CrossRef]
- Narita, H.; Tanaka, M.; Morisaku, K.; Abe, T. Extraction of Gold(III) in Hydrochloric Acid Solution Using Monoamide Compounds. Hydrometallurgy 2006, 81, 153–158. [Google Scholar] [CrossRef]
- Motokawa, R.; Kobayashi, T.; Endo, H.; Mu, J.; Williams, C.D.; Masters, A.J.; Antonio, M.R.; Heller, W.T.; Nagao, M. A Telescoping View of Solute Architectures in A Complex Fluid System. ACS Cent. Sci. 2019, 5, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Kikuchi, K.; Sugita, T.; Motokawa, R. Extraction Performance of a Fluorous Phosphate for Zr(IV) from HNO3 Solution: Comparison with Tri-n-Butyl Phosphate. Solvent Extr. Ion Exch. 2019, 37, 347–359. [Google Scholar] [CrossRef]
- Mu, J.; Motokawa, R.; Akutsu, K.; Nishitsuji, S.; Masters, A.J. A Novel Microemulsion Phase Transition: Toward the Elucidation of Third-Phase Formation in Spent Nuclear Fuel Reprocessing. J. Phys. Chem. B 2018, 122, 1439–1452. [Google Scholar] [CrossRef]
- Antonio, M.R.; Demars, T.J.; Audras, M.; Ellis, R.J. Third phase inversion, red oil formation, and multinuclear speciation of tetravalent cerium in the tri-n-butyl phosphate–n-dodecane solvent extraction system. Sep. Sci. Technol. 2018, 53, 1834–1847. [Google Scholar] [CrossRef]








| Stripping Reagent | %Stripping of Pt(IV) (%) | ||
|---|---|---|---|
| MonoAA 1 | MonoOA 2 | MonoBU 3 | |
| 5.0 M HCl | 13.8 | 9.3 | 4.5 |
| 5.0 M HNO3 | 53.4 | 81.3 | 73.7 |
| Water | 59.3 | 74.8 | 68.1 |
| 0.1 M NH3 | 60.0 | 78.8 | 70.2 |
| 0.1 M NaOH | 60.4 | 69.6 | 64.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, Y.; Morisada, S.; Kawakita, H.; Ohto, K. Selective Extraction of Platinum(IV) from the Simulated Secondary Resources Using Simple Secondary Amide and Urea Extractants. Separations 2021, 8, 139. https://doi.org/10.3390/separations8090139
Ueda Y, Morisada S, Kawakita H, Ohto K. Selective Extraction of Platinum(IV) from the Simulated Secondary Resources Using Simple Secondary Amide and Urea Extractants. Separations. 2021; 8(9):139. https://doi.org/10.3390/separations8090139
Chicago/Turabian StyleUeda, Yuki, Shintaro Morisada, Hidetaka Kawakita, and Keisuke Ohto. 2021. "Selective Extraction of Platinum(IV) from the Simulated Secondary Resources Using Simple Secondary Amide and Urea Extractants" Separations 8, no. 9: 139. https://doi.org/10.3390/separations8090139
APA StyleUeda, Y., Morisada, S., Kawakita, H., & Ohto, K. (2021). Selective Extraction of Platinum(IV) from the Simulated Secondary Resources Using Simple Secondary Amide and Urea Extractants. Separations, 8(9), 139. https://doi.org/10.3390/separations8090139