1. Introduction
Plastic debris of less than 5 mm in length is defined as microplastics (MP) [
1] and has been characterized as “extremely” persistent in the environment by the European Chemicals Agency (ECHA). Microplastics may even cause irreversible contamination and potentially threaten human health and/or the environment [
2,
3]. Therefore, the pollution with MP has not only aroused scientific interest, but has also contributed to the increased public and political awareness concerning other topics related to plastics, which has led to legal and political actions and strategic documents. In addition to this type of debris, MP released in washing PES textiles, as well as particles used in medical, industrial, and personal care products are classified as “secondary microplastics” [
4,
5].
One of the more common types of microplastics found in the environment are fibers originating from textile materials. Some research has indicated the presence of natural and synthetic fibers in the marine environment [
6,
7].
The release of micro-sized polymer particles—MP from textile materials—is an area of research that preoccupies researchers from different fields, indicating the interdisciplinarity of this topic [
8]. It has been found that the release potential of fibers depends on the donor properties of the textile material [
9], and even if fibers released from natural textile materials do not represent a significant effluent load, they can be carriers of substances that significantly burden the environment [
10].
The research of MP is relatively complex due to the size and shape of particles from various polymers, which represent serious scientific challenges. There is insufficient information on how chemical properties (additives, flame retardant agents, dyes) and the increased number of synthetic fibers and their blends with natural fibers affect the characterization of microfibers [
9].
Advances in analytical techniques for microplastics characterization have indicated the prevalence of synthetic polymers categorized in the micro (<100 μm) and nano (<100 nm) classes. However, additional data/information is needed to determine the quantities, sources, transfers, and persistence of different types of microfibers [
11]. The origin of MP is heterogeneous, and different techniques need to be used for their characterization, with thermoanalytical techniques as the most important ones. Pyrolysis combined with gas chromatography and mass spectrometry (Py-GC/MS) is used for qualitative-quantitative analysis of MP in various matrices, such as drinking water, seawater, sea salt, biosolids, sediments, and washing residues [
12,
13,
14,
15].
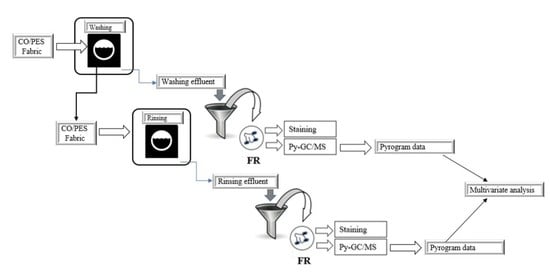
In this study, a textile composite of complex structure, made of cotton cellulose and polyester, was subjected to cyclic washing and rinsing with standard detergent and water. Separately collected effluents from the washing and rinsing cycle were filtered to separate dispersive pollutant as the filter residue (FR).
Given the polymer content in the composite of cotton cellulose and polyester, the aim of this study was to determine which component is prevalent in the filter residue (FR). To the best of our knowledge, the Py-GC/MS method as a thermal and rapid technique for the characterization of polymeric substances has not been used to date for characterization of dispersive pollutant from the cyclic washing and rinsing process.
The gravimetric analysis for the determination of the total solid substance (TSS) and staining by dyestuff as identification techniques of dispersive pollutant was applied as an auxiliary method. Further multivariate analysis of HCA based on the m/z chromatogram dataset of the related components was chosen to identify the differences in contamination with MF of washing and rinsing effluents.
3. Results and Discussion
The nature of textiles as the pollutant material and its sheddability are important criteria in environmental fiber transfer. The sheddability depends on the type of textile (woven, knitted or non-woven), the texture (open or compact), the type of yarn, and the properties of the fibers in the composite, as well as whether the fibers are cut or filaments [
9]. Blends of cotton with polyester, as well as other textile materials, release fibrils in the washing process, depending on the fabric structure and the properties of the yarn. The composite sample as shown in
Figure 1 is a contaminant material characterized by unevenness and hairiness, which may affect the separability during the washing and rinsing process. Hydrophobic polyester (PES) has better wear resistance than hydrophilic cellulose fibers [
16]. Moreover, the release is affected by the high alkalinity of the detergent solution (pH 10.3) due to the increased swelling of cotton cellulose as well as hydrolysis of PES, which further affects the migration of fibrils, i.e., fibril formation from the surface in the washing and rinsing process. This is confirmed by the results of the quantitative gravimetric analysis of FRs and TSS (
Table 2).
The TSS values in
Table 2 show the differences in FR after washing and rinsing. Higher TSS values were obtained in the rinsing process compared to the washing process. The double TSS value of FR after washing with the detergent (FR-W-D) indicates a significant influence in the bath composition on the particles effluent load.
However, the TSS of FR-R-D (72.9 mg/L) is slightly higher than the TSS of FR-R-W after rinsing with water (62.2 mg/L). These values indicate that FR-R-D contains a certain amount of detergent in combination with fibril formations, while FR-R-W contains only fibril formations.
The identification of the particle pollutant in FR was carried out through staining with dyestuffs, Telon Blue M-GLW dye for the PES component, and Bezaktiv Brilliantblau V-R spez. for cotton cellulose (
Table 3).
Photographs of the samples after staining in
Table 3 show differences in the coloring of the composite, as well as in its structural residues filter. The diverse distribution on FRs indicates the MF released from the composite. The residues on the FR-W-W are higher than FR-W-D, which indicates a higher degree of load with pollutant particles in the effluent after washing. There is a significant effect of rinsing on the structural residues released from the composites compared to washing. The results of these analyses were used as guidelines for the sampling of a representative portion of the FR for pyrolysis.
The confirmed difference in FR after staining indicates the difference in the release of fibrils from the surface of the composite material, which is due to the influence in the process variables of washing and rinsing on the sheddability of structural parameters. As can be seen from the figures, this composite sample of cotton cellulose and PES releases smaller structural units, e.g., fibers, fibrils, and fragments during the washing and rinsing process. Due to the composition of the washing bath, dissolved and/or undissolved detergent ingredients can be adsorbed on the structural units, especially since they are not aimed for stain removal, thus they are oriented to a composite sample characterized by a large active surface.
By the Py-GC/MS technique, rapid screening of the chemical composition of the sample at the molecular level is made possible, especially in the case of a mixture of complex components, by comparing chromatograms of standard polymers [
13,
14,
15,
16,
17,
18].
Given the complexity of the observed washing system, pyrolysis of detergent, composite sample, and structural units was performed. The pyrolytic degradation of the standard detergent is shown in
Figure 3 as a chromatogram with retention time.
The chemical compound as a pyrolytic product and quantitative indicators of detergents are shown in
Table 4. Proposed identification by comparing with the chromatogram of standard polymers was performed and marked with font a, b, c, d, e, f, g, h as a superscript [
19,
20,
21,
22,
23,
24,
25,
26].
Given the composition and complexity of the CO/PES composite sample, pyrolysis of the whole composite sample and its structural elements was performed, as shown in
Figure 4,
Figure 5 and
Figure 6 and
Table 5.
The pollutant material from CO/PES is a composite material, whose mechanism of pyrolytic degradation is complex due to the share of certain structural parameters of the components in the fabric. The higher the proportion of each component, the greater the defragmentation, i.e., the products of pyrolytic degradation will be observed in higher concentrations. This can be clearly seen from the formation of levoglucosan (13.65%) and benzoic acid (1.08%) in the CO/PES sample (
Table 5). It is known that in polyester the decarboxylation reaction of large polymer fragments causes the formation of benzenecarboxylic acid (benzoic acid) [
17].
The presence of benzoic acid in a concentration of 1.08% in the CO/PES sample is proof that this is a polyester-based composite material. Furthermore, the defragmented benzoic acid products are further cleaved with cotton cellulose products, creating new inter-reactions, but also reactions at ionic levels, resulting in the formation of a very large number of compounds, as shown in
Table 5. Observing the other obtained products of pyrolytic degradation,
Table 5 shows that the compounds from cotton cellulose dominate, which is expected given the composition of the tested material, in which cotton predominates (with a 60% share) compared to polyester (a 40% share). This is supported by the fact that the compound with one of the largest shares in the sample CO/PES (60/40) is levoglucosan (13.65%).
In the primary process of pyrolytic degradation of cellulose, the glycosidic bond breaks down to form levoglucosan, while on the other hand, small molecules are formed by cleavage of the pyran ring and its reformation [
18].
According to Moldoveanu [
22], levoglucosan is one of the main products of pyrolytic degradation of cellulose formed at temperatures above 400 °C. Other significant secondary compounds that can be formed during pyrolytic degradation of cellulose can be divided into several groups, such as furans (furfural), sugar anhydrides, acids, esters, aldehydes, alcohols, pyrans, ketones, aromatic hydrocarbons, and phenols [
25], some of which can be seen in
Table 5. Generally, the mechanism of pyrolytic degradation of cellulose begins with three basic competitive reactions, side group (Ei) elimination of water, chain scissions by transglycosidation, and chain scissions with reverse aldolization (retroaldolization), followed by further degradation i:
Which type of these reactions will prevail depends on a large number of factors and is difficult to predict. In general, two pyrolytic cellulose degradation reactions, depolymerization and fragmentation, are possible in parallel. The process of depolymerization of cellulose includes the formation of anhydro-oligosaccharides, monomeric anhydrosugars and derivatives (dominated by levoglucosan), furans, cyclopentanones, and other products, while fragmentation (cleavage of the ring) involves the formation of linear carbonyls, linear alcohols, linear esters, and other compounds [
27,
28].
One of the examples of depolymerization is the reaction of intramolecular elimination (Ei) of H
2O from the ring side groups with the formation of 5-hydroxymethyl-2-furancarboxaldehyde (0.51%) observed in the CO/PES sample according to reaction in
Figure 7 as demonstrated [
21,
22].
As
Figure 7 shows 5-Hydroxymethyl-2-furancarboxaldehyde is further degraded leading to the formation of furfural, which was observed in higher proportions also in samples FR-W-W (1.57%) and FR-R-W (0.70%) according to reaction in
Figure 8 [
22].
After the chromatogram analysis of the basic components of the system, the pyrolytic decomposition of FRs was performed for the purpose of a qualitative and quantitative assessment after the washing and rinsing process, depending on the bath composition. Based on the obtained mass spectra, only a part of the compounds can be reliably identified. This is due to the low concentration of some compounds, the coelution of compounds on the GC column, and the limitations of the mass spectrum database.
Pyrolysis-gas chromatography mass spectrometry and Py-GC/MS chromatograms of FR after washing and rinsing according to
Figure 2 are typically complex, with numerous compounds detected (
Table 6 and
Table 7).
In FR-W-W and FR-R-W, the proportion of 5-hydroxymethyl-2-furancarboxaldehyde increases with the rinsing process, in order that it can be assumed that rinsing supports the reaction mechanism of the intramolecular elimination (Ei) of H
2O from the ring side groups. During this process, other reactions can occur that can generate small molecules, such as formaldehyde, which was not observed in the samples, thus further degradation can be assumed. Another compound observed in
Table 5 is formic acid with a content of 15.05%. It is known that cotton cellulose dissolves into formic acid in hydrothermal conditions in concentrations of up to 1% (
w/
w), which promotes the formation of sugars and oligomers [
29]. Since in the CO/PES sample, apart from formic acid (15.05%), a high concentration of levoglucosan (13.65%) as well as oligomers were found, in this case it can also be said that in the mentioned sample the mechanism of Ei elimination of H
2O dominates. The best example is the formation of levoglucosan according to reaction in
Figure 9 [
22].
The complete dominance of the reaction mechanism of the intramolecular elimination (Ei) of H2O from the side groups of the ring can also be observed by monitoring the content of levoglucosan in the samples FR-W-W (6.38%) and FR-R-W (1.83%).
The reaction mechanism of transglycosylation can also be observed in the samples of the filter residues FR-W-W and FR-R-W, through the formation of glycolaldehyde derivatives and glycolaldehyde dimers (1,4-Dioxane-2,5-diol). After washing, the concentration (1,4-Dioxane-2,5-diol) increased from 17.31% in the FR-R-W sample to 34.07% in the FR-W-W sample, from which it can be assumed that rinsing enhances the reaction mechanism of transglycosylation. Glycolaldehyde dimer (1,4-Dioxane-2,5-diol) was also observed in the CO/PES sample (60/40) with a content of 21.16% (
Table 5).
Comparing the concentration of benzoic acid derived from polyester with the samples FR-W-W (2.17%) and FR-R-W (0.87%) in relation to the initial concentration in the sample CO/PES (1.08%) can be attributed to the decarboxylation of polyester.
2-Propanone, 1-hydroxy- (Acetol), which is one of the simple ketones, was found in higher concentrations in samples FR-W-W (5.20%) and FR-R-W (7.47%). Its formation is related to the degradation of glycerol, as in glycol aldehydes. Acetol contains both hydroxide and carbonyl functional groups, which are susceptible to decarboxylation and dehydroxylation due to pyrolysis. Samples FR-W-W and FR-R-W contain a large number of other ketones in addition to Acetol, which can be attributed to the decarboxylation process that prevails along with the defragmentation of benzoic acid. One example is the formation of β-ketonic acid in the FR-R-W sample. Pyrolysis of β-ketone acid is known to lead to CO2 elimination and ketone formation.
In the pyrolytic decomposition products of FR-W-D and FR-R-D, no levoglucosan formation was observed (
Table 7), although it was found in the composite sample and FR-R-W. The reason for the absence of levoglucosan formation may be the competitive transglycosylation reaction, with chain cleavage. Its presence in the detergent sample comes from the cellulose derivative, carboxymethylcellulose (CMC), which was adsorbed to the cotton cellulose during washing. The CMC is a component of detergent that is aimed to antiredeposition of cellulose derived textiles during the washing process [
30].
The results (
Table 7 and
Figure 10) show a significant influence in detergent on the composition of the filter residue, i.e., on the composition of released formations found in the effluent from washing and rinsing the CO/PES fabric, which confirms the previously explained TSS values.
An increased number of benzene ring-containing fragments was observed in the FR-W-D and FR-R-D samples. It can be presumed that these fragments originate from the basic raw materials for the production of LAS, which are dodecylbenzenesulfonate and chloroform [
31]. The putative mechanism of pyrolytic degradation of FR is the splitting of the alkyl chain into smaller fragments with the benzene ring. The concentration of these compounds in the samples FR-W-D and FR-R-D increases and traces of fatty acids are visible.
The investigated pollutant—CO/PES material releases microfibers (MF) in the washing and rinsing process, which is evidenced by the presented results. It can be deduced that this material has the potential to pollute wastewater with dispersed particles, of which microfibers (MF) are of particular interest. This pollutant consists of a natural polymer (cellulose) and a synthetic one (PES), which not only have different properties but also pose some risk to the environment. Released cellulose fibers are readily biodegradable but have a high degree of swelling and thus the ability to retain adsorbed substances from a complex dispersion system, such as the washing process. As a result, they can bind and retain other pollutants in the wastewater, contributing to further contamination of the system at all levels. On the other hand, the component PES is a pollutant that is difficult to biodegrade, but due to its weak hydrophilicity and swelling ability, it cannot bind and retain pollutants as cellulose MF can [
32].
Given the complexity of the system, which is manifested through the composite sample, the multi-component detergent that is not used to remove stains, high alkalinity, multiple washing and rinsing processes, there is a pronounced interaction of parameters affecting the release of fibrils or fibrous formations in washing. This research has confirmed that fragments of cotton cellulose prevail in the filter residue, which is not in line with studies claiming that PES releases more microfibers than cotton [
16]. Therefore, the potential pollution of wastewater from this system manifests itself primarily in the retention and binding of other pollutants from textile processes.
As presented in this research, numerous reactions and degradation products are not sufficient to characterize and compare these complex systems, in which washing, rinsing, and bath composition (water and/or detergent) play a significant role. Therefore, the multivariate analysis algorithm was applied to the database of chromatograms for all FRs to obtain reduced data with new information about the system. All analyses were carried out on characteristic fragmentation ions (
m/z) of datasets. HCA was performed to confirm the similarities and differences in individual FRs, and the results are shown in
Figure 11.
The obtained dendrogram shows a grouping of FRs with similar characteristics, and the belonging to the same group of FRs obtained from the washing process is visible; there is no difference with respect to the applied bath (water and/or detergent). For FRs from the rinsing process, the influence in the bath is visible, since FR-R-D shows a significant difference compared to the other samples.