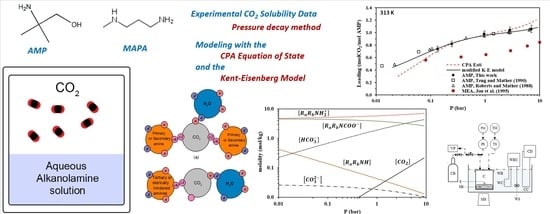
Solubility of CO2 in 2-Amino-2-methyl-1-propanol (AMP) and 3-(Methylamino)propylamine (MAPA): Experimental Investigation and Modeling with the Cubic-Plus-Association and the Modified Kent-Eisenberg Models
Abstract
:1. Introduction
| Temperature (K) | Composition | Reference |
|---|---|---|
| 313, 333, 353, 373 | 8 m | Chen et al. [26] |
| 313, 353, 393 | 1, 2 M | Arshad et al. [10] |
| 313, 333, 353, 373 | 8 m | Voice et al. [27] |
| Temperature (K) | Composition | Reference |
|---|---|---|
| 313, 393 | 3 M | Sartori and Savage [13] |
| 313, 373 | 2, 3 M | Roberts and Mather [28] |
| 323 | 3.43 M | Teng and Mather [29] |
| 313, 343 | 2 M | Teng and Mather [30] |
| 293, 313, 333, 353 | 2, 3 M | Tontiwachwuthikul et al. [31] |
| 301, 303, 313, 323, 333, 343, 353 | 2 M | Haji-sulaiman and Aroua [32] |
| 303 | 2 M | Haji-sulaiman et al. [33] |
| 313 | 2 M | Jane and [34] |
| 313, 333, 353 | 2.43, 2.44, 2.45, 6.14, 6.24, 6.48 mol/kg | Silkenbäumer et al. [35] |
| 313, 333, 353 | 30 wt. % | Park et al. [36] |
| 303, 313, 323 | 2, 2.8, 3.4 M | Kundu et al. [37] |
| 313, 333, 353, 373, 393 | 3 M | Tobiesen et al. [38] |
| 323 | 15, 30 wt. % | Arcis et al. [39] |
| 313 | 3 M | Yang et al. [40] |
| 298, 308, 318, 328 | 2.5, 3.4, 4.9 mol/dm3 | Dash et al. [41] |
| 303, 313, 333 | 1, 2, 3 M | Sharrif et al. [42] |
| 313, 333, 353, 373 | 30 wt. % | Tong et al. [43] |
| 313, 333, 363 | 2 M | Nordin et al. [44] |
| 313, 333, 353, 373 | 3.4 M, 30 wt. % | Tong et al. [45] |
| 373, 393, 413, 433 | 4.8 M | Li et al. [46] |
| 313 | 2.5 M | Nouacer et al. [47] |
| 318 | 3 M | Kortunov et al. [48] |
| 313, 333, 363 | 2 M | Narku-Tetteh et al. [49] |
| 313, 333, 353 | 0.1, 1, 3 M | Hartono et al. [50] |
2. Experimental Setup
2.1. Materials
2.2. Experimental Apparatus and Procedure
3. Theory
3.1. The Cubic Plus Association (CPA) Equation of State
3.2. The Kent-Eisenberg Model
4. Results and Discussion
4.1. Experimental Results
4.2. CPA Modeling Results
4.3. Kent-Eisenberg Model Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cannone, S.F.; Lanzini, A.; Santarelli, M. A review on CO2 capture technologies with focus on CO2-enhanced methane recovery from hydrates. Energies 2021, 14, 387. [Google Scholar] [CrossRef]
- Koukouzas, N.; Christopoulou, M.; Giannakopoulou, P.P.; Rogkala, A.; Gianni, E.; Karkalis, C.; Pyrgaki, K.; Krassakis, P.; Koutsovitis, P.; Panagiotaras, D.; et al. Current CO2 capture and storage trends in Europe in a view of social knowledge and acceptance. A short review. Energies 2022, 15, 5716. [Google Scholar] [CrossRef]
- WRI. Climate Analysis Indicators Tool; World Resources Institute: Washington, DC, USA, 2007. [Google Scholar]
- Ritchie, H.; Roser, M.; Rosado, P. CO2 and Greenhouse Gas Emissions. 2020. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 20 December 2020).
- Kenarsari, S.D.; Yang, D.; Jiang, G.; Zhang, S.; Wang, J.; Russell, A.G.; Wei, Q.; Fan, M. Review of recent advances in carbon dioxide separation and capture. RSC Adv. 2013, 3, 22739–22773. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, A.I.; Tzirakis, F.; Tsivintzelis, I.; Seferlis, P. Phase-change solvents and processes for postcombustion CO2 capture: A detailed review. Ind. Eng. Chem. Res. 2019, 58, 5088–5111. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, A.I.; Perdomo, F.A.; Tzirakis, F.; Shavalieva, G.; Tsivintzelis, I.; Kazepidis, P.; Nessi, E.; Papadokonstantakis, S.; Seferlis, P.; Galindo, A.; et al. Molecular engineering of sustainable phase-change solvents: From digital design to scaling-up for CO2 capture. Chem. Eng. J. 2021, 420, 127624. [Google Scholar] [CrossRef]
- Arshad, M.W.; Svendsen, H.F.; Fosbøl, P.L.; von Solms, N.; Thomsen, K. Equilibrium total pressure and CO2 solubility in binary and ternary aqueous solutions of 2-(diethylamino) ethanol (DEEA) and 3-(methylamino) propylamine (MAPA). J. Chem. Eng. Data 2014, 59, 764–774. [Google Scholar] [CrossRef]
- Caplow, M. Kinetics of carbamate formation and breakdown. J. Am. Chem. Soc. 1968, 90, 6795–6803. [Google Scholar] [CrossRef]
- Danckwerts, P.V. The reaction of CO2 with ethanolamines. Chem. Eng. Sci. 1979, 34, 443–446. [Google Scholar] [CrossRef]
- Sartori, G.; Savage, D.W. Sterically hindered amines for carbon dioxide removal from gases. Ind. Eng. Chem. Fundam. 1983, 22, 239–249. [Google Scholar] [CrossRef]
- Zhang, J. Study on CO2 Capture Using Thermomorphic Biphasic Solvents with Energy Efficient Regeneration. Ph.D. Thesis, Dortmund Technische Universität, Dortmund, Germany, 2014. [Google Scholar]
- Tzirakis, F.; Tsivintzelis, I.; Papadopoulos, A.I.; Seferlis, P. Experimental measurement and assessment of equilibrium behaviour for phase change solvents used in CO2 capture. Chem. Eng. Sci. 2019, 199, 20–27. [Google Scholar] [CrossRef]
- Tzirakis, F.; Tsivintzelis, I.; Papadopoulos, A.I.; Seferlis, P. Experimental investigation of phase change amine solutions used in CO2 capture applications: Systems with dimethylcyclohexylamine (DMCA) and N-cyclohexyl-1,3-propanediamine (CHAP) or 3-methylaminopropylamine (MAPA). Int. J. Greenh. Gas Control. 2021, 109, 103353. [Google Scholar] [CrossRef]
- Pinto, D.D.; Zaidy, S.A.; Hartono, A.; Svendsen, H.F. Evaluation of a phase change solvent for CO2 capture: Absorption and desorption tests. Int. J. Greenh. Gas Control. 2014, 28, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Uyan, M.; Sieder, G.; Ingram, T.; Held, C. Predicting CO2 solubility in aqueous N-methyldiethanolamine solutions with ePC-SAFT. Fluid Phase Equilib. 2015, 393, 91–100. [Google Scholar] [CrossRef]
- Wangler, A.; Sieder, G.; Ingram, T.; Heilig, M.; Held, C. Prediction of CO2 and H2S solubility and enthalpy of absorption in reacting N-methyldiethanolamine/water systems with ePC-SAFT. Fluid Phase Equilib. 2018, 461, 15–27. [Google Scholar] [CrossRef]
- Suleman, H.; Maulud, A.S.; Man, Z. Review and selection criteria of classical thermodynamic models for acid gas absorption in aqueous alkanolamines. Rev. Chem. Eng. 2015, 31, 599–639. [Google Scholar] [CrossRef]
- Rodriguez, J.; Mac Dowell, N.; Llovell, F.; Adjiman, C.S.; Jackson, G.; Galindo, A. Modelling the fluid phase behaviour of aqueous mixtures of multifunctional alkanolamines and carbon dioxide using transferable parameters with the SAFT-VR approach. Mol. Phys. 2012, 110, 1325–1348. [Google Scholar] [CrossRef] [Green Version]
- Chremos, A.; Forte, E.; Papaioannou, V.; Galindo, A.; Jackson, G.; Adjiman, C.S. Modelling the phase and chemical equilibria of aqueous solutions of alkanolamines and carbon dioxide using the SAFT-γ SW group contribution approach. Fluid Phase Equilib. 2016, 407, 280–297. [Google Scholar] [CrossRef] [Green Version]
- Leontiadis, K.; Tzimpilis, E.; Aslanidou, D.; Tsivintzelis, I. Solubility of CO2 in 3-amino-1-propanol and in N-methyldiethanolamine aqueous solutions: Experimental investigation and correlation using the CPA equation of state. Fluid Phase Equilib. 2019, 500, 112254. [Google Scholar] [CrossRef]
- Tzirakis, F.; Papadopoulos, A.I.; Seferlis, P.; Tsivintzelis, I. CO2 Solubility in diethylenetriamine (DETA) and triethylenetetramine (TETA) aqueous mixtures: Experimental investigation and correlation using the CPA equation of state. Chem. Thermodyn. Therm. Anal. 2021, 3, 100017. [Google Scholar] [CrossRef]
- Wang, T.; El Ahmar, E.; Coquelet, C.; Kontogeorgis, G.M. Improvement of the PR-CPA equation of state for modelling of acid gases solubilities in aqueous alkanolamine solutions. Fluid Phase Equilib. 2018, 471, 74–87. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Closmann, F.; Rochelle, G.T. Accurate screening of amines by the wetted wall column. Energy Procedia 2011, 4, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Voice, A.K.; Vevelstad, S.J.; Chen, X.; Nguyen, T.; Rochelle, G.T. Aqueous 3-(methylamino) propylamine for CO2 capture. Int. J. Greenh. Gas Control. 2013, 15, 70–77. [Google Scholar] [CrossRef]
- Roberts, B.E.; Mather, A.E. Solubility of CO2 and H2S in a hindered amine solution. Chem. Eng. Comm. 1988, 64, 105–111. [Google Scholar] [CrossRef]
- Teng, T.T.; Mather, A.E. Solubility of H2S, CO2 and their mixtures in an AMP solution. Can. J. Chem. Eng. 1989, 67, 846–850. [Google Scholar] [CrossRef]
- Teng, T.T.; Mather, A.E. Solubitiy of CO2 in an AMP solution. J. Chem. Eng. Data 1990, 35, 410–411. [Google Scholar] [CrossRef]
- Tontiwachwuthikul, P.; Meisen, A.; Lim, C.J. Solubility of CO2 in 2-Amino-2-methyl-1-propanol Solutions. J. Chem. Eng. Data 1991, 36, 130–133. [Google Scholar] [CrossRef]
- Haji-Sulaiman, M.Z.; Aroua, M.K. Equilibrium of CO2 in aqueous diethanolamine (DEA) and amino methyl propanol (AMP) solutions. Chem. Eng. Comm. 1995, 140, 157–171. [Google Scholar] [CrossRef]
- Haji-Sulaiman, M.Z.; Aroua, M.K.; Pervez, M.I. Equilibrium concentration profiles of species in CO2—Alkanolamine—Water systems. Gas Sep. Purif. 1996, 10, 13–18. [Google Scholar] [CrossRef]
- Jane, I.S.; Li, M.H. Solubilities of mixtures of carbon dioxide and hydrogen sulfide in water + diethanolamine + 2-amino-2-methyl-1-propanol. J. Chem. Eng. Data 1997, 42, 98–105. [Google Scholar] [CrossRef]
- Silkenbäumer, D.; Rumpf, B.; Lichtenthaler, R.N. Solubility of carbon dioxide in aqueous solutions of 2-Amino-2-methyl-1-propanol and N-Methyldiethanolamine and their mixtures in the temperature range from 313 to 353 K and pressures up to 2.7 MPa. Ind. Eng. Chem. Res. 1998, 37, 3133–3141. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, K.B.; Hyun, J.C.; Kim, S.H. Correlation and prediction of the solubility of carbon dioxide in aqueous alkanolamine and mixed alkanolamine solutions. Ind. Eng. Chem. Res. 2002, 41, 1658–1665. [Google Scholar] [CrossRef]
- Kundu, M.; Mandal, B.P.; Bandyopadhyay, S.S. Vapor—Liquid equilibrium of CO2 in aqueous solutions of 2-Amino-2-methyl-1-propanol. J. Chem. Eng. Data 2003, 48, 789–796. [Google Scholar] [CrossRef]
- Tobiesen, F.A.; Svendsen, H.F.; Mejdell, T. Modeling of blast furnace CO2 capture using amine absorbents. Ind. Eng. Chem. Res. 2007, 46, 7811–7819. [Google Scholar] [CrossRef]
- Arcis, H.; Rodier, L.; Coxam, J.Y. Enthalpy of solution of CO2 in aqueous solutions of 2-amino-2-methyl-1-propanol. J. Chem. Thermodyn. 2007, 39, 878–887. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Soriano, A.N.; Caparanga, A.R.; Li, M.H. Equilibrium solubility of carbon dioxide in (2-amino-2-methyl-1-propanol + piperazine + water). J. Chem. Thermodyn. 2010, 42, 659–665. [Google Scholar] [CrossRef]
- Dash, S.K.; Samanta, A.N.; Bandyopadhyay, S.S. (Vapour + liquid) equilibria (VLE) of CO2 in aqueous solutions of 2-amino-2-methyl-1-propanol: New data and modelling using eNRTL-equation. J. Chem. Thermodyn. 2011, 43, 1278–1285. [Google Scholar] [CrossRef]
- Shariff, A.M.; Murshid, G.G.; Lau, K.K.; Bustam, M.A.; Ahmad, F. Solubility of CO2 in aqueous solutions of 2-amino-2-methyl-1-propanol at high pressure. Int. J. Environ. Ecol. Eng. 2011, 5, 825–828. [Google Scholar]
- Tong, D.; Trusler, J.M.; Maitland, G.C.; Gibbins, J.; Fennell, P.S. Solubility of carbon dioxide in aqueous solution of monoethanolamine or 2-amino-2-methyl-1-propanol: Experimental measurements and modelling. Int. J. Greenh. Gas Control. 2012, 6, 37–47. [Google Scholar] [CrossRef]
- Nordin, S.; Salleh, R.M.; Ismail, N. 2013 experimental study on CO2 absorption in aqueous mixtures of 2-amino-2-methyl-1-propanol and N-butyl-3-methylpyridinium tetrafluoroborate. In Proceedings of the IEEE Business Engineering and Industrial Applications Colloquium (BEIAC), Langkawi, Malaysia, 7–9 April 2013; pp. 549–552. [Google Scholar]
- Tong, D.; Maitland, G.C.; Trusler, M.J.; Fennell, P.S. Solubility of carbon dioxide in aqueous blends of 2-amino-2-methyl-1-propanol and piperazine. Chem. Eng. Sci. 2013, 101, 851–864. [Google Scholar] [CrossRef]
- Li, H.; Frailie, P.T.; Rochelle, G.T.; Chen, J. Thermodynamic modeling of piperazine/2-aminomethylpropanol/CO2/water. Chem. Eng. Sci. 2014, 117, 331–341. [Google Scholar] [CrossRef]
- Nouacer, A.; Belaribi, F.B.; Mokbel, I.; Jose, J. Solubility of carbon dioxide gas in some 2.5 M tertiary amine aqueous solutions. J. Mol. Liq. 2014, 190, 68–73. [Google Scholar] [CrossRef]
- Kortunov, P.V.; Siskin, M.; Paccagnini, M.; Thomann, H. CO2 reaction mechanisms with hindered alkanolamines: Control and promotion of reaction pathways. Energy Fuels 2016, 30, 1223–1236. [Google Scholar]
- Narku-Tetteh, J.; Muchan, P.; Saiwan, C.; Supap, T.; Idem, R. Selection of components for formulation of amine blends for post combustion CO2 capture based on the side chain structure of primary, secondary and tertiary amines. Chem. Eng. Sci. 2017, 170, 542–560. [Google Scholar] [CrossRef]
- Hartono, A.; Ahmad, R.; Usman, M.; Asif, N.; Svendsen, H.F. Solubility of CO2 in 0.1 M, 1 M and 3 M of 2-amino-2-methyl-1-propanol (AMP) from 313 to 393K and model representation using the eNRTL framework. Fluid Phase Equilib. 2020, 511, 112485. [Google Scholar] [CrossRef]
- Kent, R.L.; Eisenberg, B. Better data for amine treating. Hydrocarb. Process 1976, 55, 87–90. [Google Scholar]
- Jou, F.-Y.; Mather, A.E.; Otto, F.D. Solubility of H2S and CO2 in aqueous methyldiethanolamine solutions. Ind. Eng. Chem. Proc. Des. Dev. 1982, 21, 539–544. [Google Scholar] [CrossRef]
- Suleman, H.; Maulud, A.S.; Fosbøl, P.L.; Nasir, Q.; Nasir, R.; Shahid, M.Z.; Nawaz, M.; Abunowara, M. A review of semi-empirical equilibrium models for CO2-alkanolamine-H2O solutions and their mixtures at high pressure. J. Environ. Chem. Eng. 2021, 9, 104713. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST). Isothermal Properties for Carbon Dioxide. Available online: https://webbook.nist.gov/chemistry/ (accessed on 31 May 2019).
- Jou, F.Y.; Mather, A.E.; Otto, F.D. The solubility of CO2 in a 30 mass percent monoethanolamine solution. Can. J. Chem. Eng. 1995, 73, 140–147. [Google Scholar] [CrossRef]
- Wertheim, M.S. Fluids with highly directional attractive forces. III. Multiple attraction sites. J. Stat. Phys. 1986, 42, 459–476. [Google Scholar] [CrossRef]
- Kontogeorgis, G.M.; Voutsas, E.C.; Yakoumis, I.V.; Tassios, D.P. An equation of state for associating fluids. Ind. Eng. Chem. Res. 1996, 35, 4310–4318. [Google Scholar] [CrossRef]
- Kontogeorgis, G.M.; Michelsen, M.L.; Folas, G.K.; Derawi, S.; Von Solms, N.; Stenby, E.H. Ten years with the CPA (Cubic-Plus-Association) equation of state. Part 2. Cross-associating and multicomponent systems. Ind. Eng. Chem. Res. 2006, 45, 4869–4878. [Google Scholar] [CrossRef]
- Tsivintzelis, I.; Kontogeorgis, G.M.; Michelsen, M.L.; Stenby, E.H. Modeling phase equilibria for acid gas mixtures using the CPA equation of state. I. Mixtures with H2S. AIChE J. 2010, 56, 2965–2982. [Google Scholar] [CrossRef]
- Haji-Sulaiman, M.Z.; Aroua, M.K.; Benamor, A. Analysis of equilibrium data of CO2 in aqueous solutions of diethanolamine (DEA), methyldiethanolamine (MDEA) and their mixtures using the modified Kent Eisenberg model. Chem. Eng. Res. Des. 1998, 76, 961–968. [Google Scholar] [CrossRef]
- Tsivintzelis, I.; Kontogeorgis, G.M.; Michelsen, M.L.; Stenby, E.H. Modeling phase equilibria for acid gas mixtures using the CPA equation of state. Part II: Binary mixtures with CO2. Fluid Phase Equilib. 2011, 306, 38–56. [Google Scholar] [CrossRef]
- Klepácová, K.; Huttenhuis, P.J.; Derks, P.W.; Versteeg, G.F. Vapor pressures of several commercially used alkanolamines. J. Chem. Eng. Data 2011, 56, 2242–2248. [Google Scholar] [CrossRef] [Green Version]
- Barreau, A.; Mougin, P.; Lefebvre, C.; Luu Thi, Q.M.; Rieu, J. Ternary isobaric vapor—Liquid equilibria of methanol + N-methyldiethanolamine + water and methanol + 2-amino-2-methyl-1-propanol + water systems. J. Chem. Eng. Data 2007, 52, 769–773. [Google Scholar] [CrossRef]
- Belabbaci, A.; Ahmed, N.C.B.; Mokbel, I.; Negadi, L. Investigation of the isothermal (vapour + liquid) equilibria of aqueous 2-amino-2-methyl-1-propanol (AMP), N-benzylethanolamine, or 3-dimethylamino-1-propanol solutions at several temperatures. J. Chem. Thermodyn. 2010, 42, 1158–1162. [Google Scholar] [CrossRef]
- Pappa, G.D.; Anastasi, C.; Voutsas, E.C. Measurement and thermodynamic modeling of the phase equilibrium of aqueous 2-amino-2-methyl-1-propanol solutions. Fluid Phase Equilib. 2006, 243, 193–197. [Google Scholar] [CrossRef]
- Li, M.H.; Lie, Y.C. Densities and viscosities of solutions of monoethanolamine + N-methyldiethanolamine + water and monoethanolamine + 2-amino-2-methyl-1-propanol + water. J. Chem. Eng. Data 1994, 39, 444–447. [Google Scholar] [CrossRef]
- Kim, I.; Svendsen, H.F.; Børresen, E. Ebulliometric determination of vapor−liquid equilibria for pure water, monoethanolamine, n-methyldiethanolamine, 3-(methylamino)-propylamine, and their binary and ternary solutions. J. Chem. Eng. Data 2008, 53, 2521–2531. [Google Scholar] [CrossRef]
- Pinto, D.D.; Monteiro, J.G.S.; Johnsen, B.; Svendsen, H.F.; Knuutila, H. Density measurements and modelling of loaded and unloaded aqueous solutions of MDEA (N-methyldiethanolamine), DMEA (N,N-dimethylethanolamine), DEEA (diethylethanolamine) and MAPA (N-methyl-1,3-diaminopropane). Int. J. Greenh. Gas Control. 2014, 25, 173–185. [Google Scholar] [CrossRef]
- Constantinou, L.; Gani, R. New group contribution method for estimating properties of pure compounds. AIChE J. 1994, 40, 1697–1710. [Google Scholar] [CrossRef]
- Tzirakis, F.; Papadopoulos, A.I.; Seferlis, P.; Tsivintzelis, I. CO2 solubility in aqueous methylcyclohexylamine (MCA) and N-cyclohexyl-1,3-propanediamine (CHAP) solutions. AIChE J. 2022; submitted for publication. [Google Scholar]
- Edwards, T.J.; Maurer, G.; Newman, J.; Prausnitz, J.M. Vapor-liquid equilibria in multicomponent aqueous solutions of volatile weak electrolytes. AIChE J. 1978, 24, 966–976. [Google Scholar] [CrossRef]













| Product Name | Abbreviation | CAS-Number | Purity | Supplier |
|---|---|---|---|---|
| Carbon dioxide | CO2 | 124-38-9 | 99.9% | Air Liquide |
| 2-Amino-2-methyl-1-propanol | AMP | 124-68-5 | 99.0% | Aldrich |
| 3-(Methylamino)propylamine | MAPA | 6291-84-5 | 97.0% | Sigma-Aldrich |
| Water | H2O | 7732-18-5 | HPLC grade | Chem lab |
| Parameters | |
|---|---|
| A | 1 |
| B | |
| C 1 | |
| D 1 | |
| E 1 | |
| F 1 |
| Parameters | |
|---|---|
| A | 1 |
| B | |
| C | |
| D | 2 [CO2] [CO2] |
| E | [CO2] |
| (bar) b | (mol CO2/mol AMP) | (bar) b | (mol CO2/mol AMP) |
|---|---|---|---|
| 298.15 K | 323.15 K | ||
| 0.10 | 0.74 ± 0.05 | 0.22 | 0.72 ± 0.05 |
| 0.15 | 0.83 ± 0.04 | 0.38 | 0.80 ± 0.05 |
| 0.76 | 1.00 ± 0.05 | 1.17 | 0.95 ± 0.05 |
| 2.23 | 1.11 ± 0.05 | 2.97 | 1.03 ± 0.05 |
| 6.41 | 1.11± 0.06 | 7.36 | 1.05 ± 0.06 |
| 313.15 K | 333.15 K | ||
| 0.13 | 0.74 ± 0.05 | 0.35 | 0.71 ± 0.05 |
| 0.24 | 0.82 ± 0.04 | 0.55 | 0.78 ± 0.05 |
| 0.96 | 0.97 ± 0.05 | 1.40 | 0.92 ± 0.05 |
| 2.85 | 1.04 ± 0.05 | 3.17 | 1.02 ± 0.05 |
| 7.07 | 1.06 ± 0.06 | 7.69 | 1.04 ± 0.06 |
| (bar) b | (mol CO2/mol MAPA) | (bar) b | (mol CO2/mol AMP) |
|---|---|---|---|
| 298.15 K | 323.15 K | ||
| 0.07 | 1.00 ± 0.02 | 0.10 | 1.00 ± 0.02 |
| 0.16 | 1.10 ± 0.02 | 0.34 | 1.09 ± 0.02 |
| 0.25 | 1.14 ± 0.02 | 0.54 | 1.12 ± 0.02 |
| 0.28 | 1.17 ± 0.02 | 0.69 | 1.15 ± 0.02 |
| 0.59 | 1.22 ± 0.02 | 1.23 | 1.18 ± 0.02 |
| 0.73 | 1.26 ± 0.02 | 1.56 | 1.21 ± 0.02 |
| 0.80 | 1.25 ± 0.02 | 1.63 | 1.20 ± 0.02 |
| 1.92 | 1.34 ± 0.02 | 3.24 | 1.27 ± 0.02 |
| 3.48 | 1.43 ± 0.02 | 5.27 | 1.34 ± 0.02 |
| 4.86 | 1.47 ± 0.02 | 6.89 | 1.37 ± 0.02 |
| 313.15 K | 333.15 K | ||
| 0.10 | 1.00 ± 0.02 | 0.12 | 1.00 ± 0.02 |
| 0.24 | 1.10 ± 0.02 | 0.47 | 1.08 ± 0.02 |
| 0.42 | 1.13 ± 0.02 | 0.75 | 1.11 ± 0.02 |
| 0.51 | 1.16 ± 0.02 | 0.91 | 1.13 ± 0.02 |
| 1.00 | 1.19 ± 0.02 | 1.55 | 1.17 ± 0.02 |
| 1.22 | 1.23 ± 0.02 | 1.93 | 1.19 ± 0.02 |
| 1.30 | 1.22 ± 0.02 | 1.99 | 1.18 ± 0.02 |
| 2.73 | 1.30 ± 0.02 | 3.77 | 1.24 ± 0.02 |
| 4.73 | 1.36 ± 0.02 | 5.96 | 1.30 ± 0.02 |
| 6.18 | 1.40 ± 0.02 | 7.73 | 1.33 ± 0.03 |
| a (K) | a0 (L2∙bar∙mol−2) | b (L∙mol−1) | c1 | εAiBi (bar∙L∙mol−1) | βAiBi | Association Scheme | Reference | |
|---|---|---|---|---|---|---|---|---|
| CO2 | 304.2 | 3.5079 | 0.0272 | 0.7602 | - | - | - | Tsivintzelis et al. [61] |
| H2O | 647.3 | 1.2277 | 0.0145 | 0.6736 | 166.55 | 0.0692 | 4C | Tsivintzelis et al. [61] |
| AMP | 619.8 b | 19.6710 | 0.0882 | 0.9050 | 162.24 | 0.0087 | 4C | This study |
| MAPA | 601.9 c | 18.2771 | 0.0929 | 1.0732 | 86.64 | 0.0557 | 4C | This study |
| kij | εcross (bar∙L∙mol−1) | βcross | εcross (bar∙L∙mol−1) | βcross | |
|---|---|---|---|---|---|
| CO2–water | 0.11406 a | Exp. 142.0 a | mCR1: 0.0162 a | - | - |
| AMP–water | −0.0325 | CR-1 | CR-1 | - | - |
| MAPA–water | −0.3364 | CR-1 | CR-1 | ||
| CO2–AMP | −0.1780 | 318.9 | 0.02184 | - | - |
| CO2–MAPA | 0.3310 | 435.3 | 0.0005 | 311.9 | 0.002 |
| Composition (M) | Temperature (K) | No. of Data Points | CPA AAD % | K-E Model AAD % |
|---|---|---|---|---|
| 2 | 298.15 | 5 | 33.9 | 6.8 |
| 2 | 313.15 | 5 | 18.1 | 1.7 |
| 2 | 323.15 | 5 | 14.1 | 2.1 |
| 2 | 333.15 | 5 | 14.7 | 4.0 |
| Total | 20.2 | 3.7 |
| Composition (M) | Temperature (K) | No. of Data Points | CPA AAD % | K-E Model AAD % |
|---|---|---|---|---|
| 3.35 | 298.15 | 10 | 26.2 | 1.5 |
| 3.35 | 313.15 | 10 | 22.1 | 1.2 |
| 3.35 | 323.15 | 10 | 20.2 | 0.7 |
| 3.35 | 333.15 | 10 | 28.7 | 0.5 |
| Total | 24.3 | 1.0 |
| Parameter (T in K) | Units | A | B | C | D | Regression Range (K) | Reference |
|---|---|---|---|---|---|---|---|
| −5936.63 | 0 | 0 | −3.1347 | 313–383 | This work | ||
| −6164.85 | 0 | 0 | −4.1080 | 313–383 | This work | ||
| −3534.70 | 0 | 0 | 7.4398 | 313–383 | This work | ||
| −12,092.10 | −36.7816 | 0 | 235.482 | 273–498 | Edwards et al. [71] | ||
| −12,431.70 | −35.4819 | 0 | 220.067 | 273–498 | Edwards et al. [71] | ||
| −13,445.90 | −22.4773 | 0 | 140.932 | 273–498 | Edwards et al. [71] | ||
| −6789.04 | −11.4519 | −0.010454 | 94.4914 | 273–498 | Edwards et al. [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontos, G.; Soldatou, M.A.; Tzimpilis, E.; Tsivintzelis, I. Solubility of CO2 in 2-Amino-2-methyl-1-propanol (AMP) and 3-(Methylamino)propylamine (MAPA): Experimental Investigation and Modeling with the Cubic-Plus-Association and the Modified Kent-Eisenberg Models. Separations 2022, 9, 338. https://doi.org/10.3390/separations9110338
Kontos G, Soldatou MA, Tzimpilis E, Tsivintzelis I. Solubility of CO2 in 2-Amino-2-methyl-1-propanol (AMP) and 3-(Methylamino)propylamine (MAPA): Experimental Investigation and Modeling with the Cubic-Plus-Association and the Modified Kent-Eisenberg Models. Separations. 2022; 9(11):338. https://doi.org/10.3390/separations9110338
Chicago/Turabian StyleKontos, Giannis, Maria Anna Soldatou, Evangelos Tzimpilis, and Ioannis Tsivintzelis. 2022. "Solubility of CO2 in 2-Amino-2-methyl-1-propanol (AMP) and 3-(Methylamino)propylamine (MAPA): Experimental Investigation and Modeling with the Cubic-Plus-Association and the Modified Kent-Eisenberg Models" Separations 9, no. 11: 338. https://doi.org/10.3390/separations9110338
APA StyleKontos, G., Soldatou, M. A., Tzimpilis, E., & Tsivintzelis, I. (2022). Solubility of CO2 in 2-Amino-2-methyl-1-propanol (AMP) and 3-(Methylamino)propylamine (MAPA): Experimental Investigation and Modeling with the Cubic-Plus-Association and the Modified Kent-Eisenberg Models. Separations, 9(11), 338. https://doi.org/10.3390/separations9110338