Polyaniline/Glauconite Nanocomposite Adsorbent for Congo Red Dye from Textile Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials, Dyes, and Reagents
2.2. Preparation of Gl/PAN Composite
2.3. Preparation of Adsorbate
2.4. Adsorption Studies
2.5. Adsorption Isotherm
2.6. Adsorption Kinetics and Mechanism
2.7. Statistical Analysis
3. Results and Discussion
3.1. Adsorbent Characterization
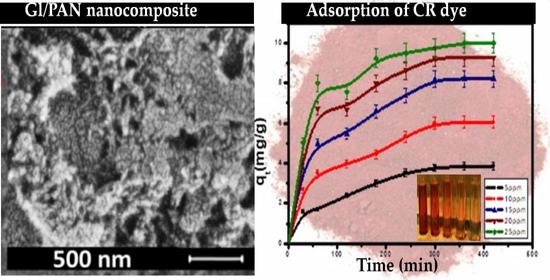
3.1.1. SEM Characterization
3.1.2. FT-IR Analysis
3.1.3. X-ray Diffraction Characterization
3.2. Factors Influencing the Adsorption Process
3.2.1. Effect of Initial Dye Concentration
3.2.2. Effect of Adsorbent Doses
3.2.3. Effect of Solution pH
3.2.4. Effect of Temperature
3.2.5. Reusability of Adsorbents
3.3. Adsorption Isotherm
3.4. Adsorption Kinetics
3.5. Sorption Mechanism
3.6. Comparison of Adsorption Capability of Gl and Gl/PAN with Other Adsorbents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Gl | Glauconite |
| Gl/PAN | Glauconite/Polyaniline composite |
| CR | Congo Red dye |
| AN | Aniline |
| PPS | Potassium per sulfate |
| HCl | Hydrochloride acid |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| FT-IR | Fourier Transformer-Infrared Spectrometer |
| pHzpc | pH at zero point charge |
| ppm | Parts Per Million (1 milligram per liter) |
References
- Sonune, A.; Ghate, R. Developments in wastewater treatment methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Campkin, B.; Cox, R. Dirt: New Geographies of Cleanliness and Contamination; IB Tauris: London, UK, 2007. [Google Scholar]
- Rathoure, A.K.; Dhatwalia, V.K. Toxicity and Waste Management Using Bioremediation; Engineering Science Reference; IGI Global USA: Hershey, PA, USA, 2016. [Google Scholar]
- Cox, M.; Négré, P.; Yurramendi, L. Industrial Liquid Effluents; INASMET Tecnalia: San Sebastian, Spain, 2007; p. 283. [Google Scholar]
- Khalaf, M.N. Green Polymers and Environmental Pollution Control; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Morin-Crini, N.; Crini, G.; Roy, L. Eaux industrielles contaminées. PUFC Besançon 2017, 513, 37–47. [Google Scholar]
- Mustapha, S.; Ndamitso, M.; Abdulkareem, A.; Tijani, J.; Shuaib, D.; Ajala, A.; Mohammed, A. Application of TiO2 and ZnO nanoparticles immobilized on clay in wastewater treatment: A review. Appl. Water Sci. 2020, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, R.; Bhaduri, D.; Sarkar, B.; Rusmin, R.; Hou, D.; Khanam, R.; Sarkar, S.; Biswas, J.K.; Vithanage, M.; Bhatnagar, A. Clay–polymer nanocomposites: Progress and challenges for use in sustainable water treatment. J. Hazard. Mater. 2020, 383, 121125. [Google Scholar] [CrossRef]
- Sokolowska-Gajda, J.; Freeman, H.S.; Reife, A. Synthetic dyes based on environmental considerations. Part 2: Iron complexes formazan dyes. Dyes Pigment. 1996, 30, 1–20. [Google Scholar] [CrossRef]
- Ivanov, K.; Gruber, E.; Schempp, W.; Kirov, D. Possibilities of using zeolite as filler and carrier for dyestuffs in paper. Papier 1996, 50, 56–60. [Google Scholar]
- Kabdaşli, I.; Tünay, O.; Orhon, D. Wastewater control and management in a leather tanning district. Water Sci. Technol. 1999, 40, 261–267. [Google Scholar] [CrossRef]
- Bensalah, N.; Alfaro, M.Q.; Martínez-Huitle, C. Electrochemical treatment of synthetic wastewaters containing Alphazurine A dye. Chem. Eng. J. 2009, 149, 348–352. [Google Scholar] [CrossRef]
- Wróbel, D.; Boguta, A.; Ion, R.M. Mixtures of synthetic organic dyes in a photoelectrochemical cell. J. Photochem. Photobiol. A Chem. 2001, 138, 7–22. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K.; Phan, C. Synthesis and characterisation of novel-activated carbon from waste biomass pine cone and its application in the removal of congo red dye from aqueous solution by adsorption. Water Air Soil Pollut. 2014, 225, 1818. [Google Scholar] [CrossRef]
- Benefield, L.D.; Judkins, J.F.; Weand, B.L. Process Chemistry for Water and Wastewater Treatment; Prentice Hall: Hoboken, NJ, USA, 1982. [Google Scholar]
- Liu, D.H.; Lipták, B.G. Hazardous Waste and Solid; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Henze, M.; Harremoes, P.; la Cour Jansen, J.; Arvin, E. Wastewater Treatment: Biological and Chemical Processes, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Harvey, P.J.; Campanella, B.F.; Castro, P.M.; Harms, H.; Lichtfouse, E.; Schäffner, A.R.; Smrcek, S.; Werck-Reichhart, D. Phytoremediation of polyaromatic hydrocarbons, anilines and phenols. Environ. Sci. Pollut. Res. 2002, 9, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Hamd, A.; Shaban, M.; AlMohamadi, H.; Dryaz, A.R.; Ahmed, S.A.; Al-Ola, K.A.A.; El-Mageed, H.R.A.; Soliman, N.K. Novel Wastewater Treatment by Using Newly Prepared Green Seaweed–Zeolite Nanocomposite. ACS Omega 2022, 7, 11044–11056. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, Y.; Sreedhara Chary, N.; Samuel Suman Raj, D. Decolourization of industrial effluents–available methods and emerging technologies—A review. Rev. Environ. Sci. Bio Technol. 2005, 4, 245–273. [Google Scholar] [CrossRef]
- Hai, F.I.; Yamamoto, K.; Fukushi, K. Hybrid treatment systems for dye wastewater. Crit. Rev. Environ. Sci. Technol. 2007, 37, 315–377. [Google Scholar] [CrossRef]
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, H.; Liu, G.; Qiao, J.; Wang, J.; Lu, H.; Yang, L.; Wu, Y. Methylene blue adsorption onto swede rape straw (Brassica napus L.) modified by tartaric acid: Equilibrium, kinetic and adsorption mechanisms. Bioresour. Technol. 2012, 125, 138–144. [Google Scholar] [CrossRef]
- Ghosh, R.K.; Reddy, D.D. Crop Residue Ashes as Adsorbents for Basic Dye (M ethylene Blue) Removal: Adsorption Kinetics and Dynamics. CLEAN—Soil Air Water 2014, 42, 1098–1105. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.; Nikkar, H.; Mahmoodi, N.; Markazi, M.; Menger, F. The sorption of cationic dyes onto kaolin: Kinetic, isotherm and thermodynamic studies. Desalination 2011, 266, 274–280. [Google Scholar] [CrossRef]
- Gupta, V. Application of low-cost adsorbents for dye removal–a review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Parab, H.; Sudersanan, M.; Shenoy, N.; Pathare, T.; Vaze, B. Use of agro-industrial wastes for removal of basic dyes from aqueous solutions. CLEAN—Soil Air Water 2009, 37, 963–969. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Tyagi, I.; Chandra, R.; Gupta, V.K. Adsorptive removal of Pb (II) ions from aqueous solution using CuO nanoparticles synthesized by sputtering method. J. Mol. Liq. 2017, 225, 936–944. [Google Scholar] [CrossRef]
- Van der Merwe, D.; Pickrell, J.A. Toxicity of Nanomaterials. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 319–326. [Google Scholar]
- Roy, R.; Roy, R.A.; Roy, D.M. Alternative perspectives on “quasi-crystallinity”: Non-uniformity and nanocomposites. Mater. Lett. 1986, 4, 323–328. [Google Scholar] [CrossRef]
- Hajjaoui, H.; Soufi, A.; Boumya, W.; Abdennouri, M.; Barka, N. Polyaniline/Nanomaterial Composites for the Removal of Heavy Metals by Adsorption: A Review. J. Compos. Sci. 2021, 5, 233. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, S.; Lu, K.; Jia, H.; Xia, M.; Wang, F. Preparation of hierarchically floral ZIF-8 derived carbon@polyaniline@Ni/Al layered double hydroxides composite with outstanding removal phenomenon for saccharin. Chem. Eng. J. 2022, 450, 138127. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, S.; Xia, M.; Wang, F.; Ju, X. Three-dimension hierarchical composite via in-situ growth of Zn/Al layered double hydroxide plates onto polyaniline-wrapped carbon sphere for efficient naproxen removal. J. Hazard. Mater. 2022, 423, 127192. [Google Scholar] [CrossRef]
- Zehhaf, A.; Benyoucef, A.; Quijada, C.; Taleb, S.; Morallon, E. Algerian natural montmorillonites for arsenic (III) removal in aqueous solution. Int. J. Environ. Sci. Technol. 2015, 12, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Franus, M.; Bandura, L.; Madej, J. Mono and poly-cationic adsorption of heavy metals using natural glauconite. Minerals 2019, 9, 470. [Google Scholar] [CrossRef] [Green Version]
- Selby, D. U-Pb zircon geochronology of the Aptian/Albian boundary implies that the GL-O international glauconite standard is anomalously young. Cretac. Res. 2009, 30, 1263–1267. [Google Scholar] [CrossRef]
- Sobeih, M.M.; El-Shahat, M.; Osman, A.; Zaid, M.; Nassar, M.Y. Glauconite clay-functionalized chitosan nanocomposites for efficient adsorptive removal of fluoride ions from polluted aqueous solutions. RSC Adv. 2020, 10, 25567–25585. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, J.; Polyaniline, G.R.G. Preparation of a conducting polymer (IUPAC technical report). Pure Appl. Chem. 2002, 74, 857–867. [Google Scholar] [CrossRef] [Green Version]
- Bhadra, S.; Singha, N.K.; Khastgir, D. Electrochemical synthesis of polyaniline and its comparison with chemically synthesized polyaniline. J. Appl. Polym. Sci. 2007, 104, 1900–1904. [Google Scholar] [CrossRef]
- Khedr, M.; Halim, K.A.; Soliman, N. Synthesis and photocatalytic activity of nano-sized iron oxides. Mater. Lett. 2009, 63, 598–601. [Google Scholar] [CrossRef]
- Soliman, N.k.; Moustafa, A.F.; Aboud, A.A.; Halim, K.S.A. Effective utilization of Moringa seeds waste as a new green environmental adsorbent for removal of industrial toxic dyes. J. Mater. Res. Technol. 2019, 8, 1798–1808. [Google Scholar] [CrossRef]
- Kausar, A.; Sher, F.; Hazafa, A.; Javed, A.; Sillanpää, M.; Iqbal, M. Biocomposite of sodium-alginate with acidified clay for wastewater treatment: Kinetic, equilibrium and thermodynamic studies. Int. J. Biol. Macromol. 2020, 161, 1272–1285. [Google Scholar] [CrossRef]
- Wang, S.G.; Liu, X.W.; Gong, W.X.; Nie, W.; Gao, B.Y.; Yue, Q.Y. Adsorption of fulvic acids from aqueous solutions by carbon nanotubes, Journal of Chemical Technology & Biotechnology: International Research in Process. Environ. Clean Technol. 2007, 82, 698–704. [Google Scholar]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Li, Z.; Chang, P.-H.; Jiang, W.-T.; Jean, J.-S.; Hong, H. Mechanism of methylene blue removal from water by swelling clays. Chem. Eng. J. 2011, 168, 1193–1200. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Temkin, M. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim. URSS 1940, 12, 327–356. [Google Scholar]
- Foo, K.; Hameed, B. Preparation, characterization and evaluation of adsorptive properties of orange peel based activated carbon via microwave induced K2CO3 activation. Bioresour. Technol. 2012, 104, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.A.; Vemparala, B.; Madras, G. Adsorption kinetics of dyes and their mixtures with Co3O4–ZrO2 composites. J. Environ. Chem. Eng. 2015, 3, 2684–2696. [Google Scholar] [CrossRef]
- Ozdemir, O.; Armagan, B.; Turan, M.; Celik, M.S. Comparison of the adsorption characteristics of azo-reactive dyes on mezoporous minerals. Dyes Pigment. 2004, 62, 49–60. [Google Scholar] [CrossRef]
- Ho, Y.; McKay, G. Comparative sorption kinetic studies of dye and aromatic compounds onto fly ash. J. Environ. Sci. Health Part A 1999, 34, 1179–1204. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ismadji, S.; Bhatia, S. A modified pore-filling isotherm for liquid-phase adsorption in activated carbon. Langmuir 2001, 17, 1488–1498. [Google Scholar] [CrossRef]
- Yuh-Shan, H. Citation review of lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Essandoh, M.; Kunwar, B.; Pittman, C.U., Jr.; Mohan, D.; Mlsna, T. Sorptive removal of salicylic acid and ibuprofen from aqueous solutions using pine wood fast pyrolysis biochar. Chem. Eng. J. 2015, 265, 219–227. [Google Scholar] [CrossRef]
- Gan, C.; Liu, Y.; Tan, X.; Wang, S.; Zeng, G.; Zheng, B.; Li, T.; Jiang, Z.; Liu, W. Effect of porous zinc–biochar nanocomposites on Cr (VI) adsorption from aqueous solution. RSC Adv. 2015, 5, 35107–35115. [Google Scholar] [CrossRef]
- Belousov, P.; Semenkova, A.; Egorova, T.; Romanchuk, A.; Zakusin, S.; Dorzhieva, O.; Tyupina, E.; Izosimova, Y.; Tolpeshta, I.; Chernov, M. Cesium sorption and desorption on glauconite, bentonite, zeolite, and diatomite. Minerals 2019, 9, 625. [Google Scholar] [CrossRef] [Green Version]
- Mostafaei, A.; Zolriasatein, A. Synthesis and characterization of conducting polyaniline nanocomposites containing ZnO nanorods. Prog. Nat. Sci. Mater. Int. 2012, 22, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Younes, H.; El-Etriby, H.K.; Mahanna, H. High removal efficiency of reactive yellow 160 dye from textile wastewater using natural and modified glauconite. Int. J. Environ. Sci. Technol. 2022, 19, 5659–5674. [Google Scholar] [CrossRef]
- Younes, H.; Mahanna, H.; El-Etriby, H.K. Fast adsorption of phosphate (PO4−) from wastewater using glauconite. Water Sci. Technol. 2019, 80, 1643–1653. [Google Scholar] [CrossRef]
- Shekhar, S.; Mishra, D.; Agrawal, A.; Sahu, K. Physical and chemical characterization and recovery of potash fertilizer from glauconitic clay for agricultural application. Appl. Clay Sci. 2017, 143, 50–56. [Google Scholar] [CrossRef]
- Selim, K.; Youssef, M.; El-Rahiem, F.A.; Hassan, M. Dye removal using some surface modified silicate minerals. Int. J. Min. Sci. Technol. 2014, 24, 183–189. [Google Scholar] [CrossRef]
- Borth, K.W.; Galdino, C.W.; de Carvalho Teixeira, V.; Anaissi, F.J. Iron oxide nanoparticles obtained from steel waste recycling as a green alternative for Congo red dye fast adsorption. Appl. Surf. Sci. 2021, 546, 149126. [Google Scholar] [CrossRef]
- Sharma, Y.C. Optimization of parameters for adsorption of methylene blue on a low-cost activated carbon. J. Chem. Eng. Data 2010, 55, 435–439. [Google Scholar] [CrossRef]
- Vikas, B.; Fasullo, M. (Eds.) Cell Growth; IntechOpen: London, UK, 2020. [Google Scholar]
- Pons, M.P.; Fuste, M.C. Uranium uptake by immobilized cells of Pseudomonas strain EPS 5028. Appl. Microbiol. Biotechnol. 1993, 39, 661–665. [Google Scholar] [CrossRef]
- Tahir, M.A.; Bhatti, H.N.; Iqbal, M. Solar Red and Brittle Blue direct dyes adsorption onto Eucalyptus angophoroides bark: Equilibrium, kinetics and thermodynamic studies. J. Environ. Chem. Eng. 2016, 4, 2431–2439. [Google Scholar] [CrossRef]
- Mohamed, H.S.; Soliman, N.; Abdelrheem, D.A.; Ramadan, A.A.; Elghandour, A.H.; Ahmed, S.A. Adsorption of Cd2+ and Cr3+ ions from aqueous solutions by using residue of Padina gymnospora waste as promising low-cost adsorbent. Heliyon 2019, 5, e01287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, R.; Ding, Y.; Liu, H.; Chen, Q.; Liu, Z. Lead biosorption and desorption by intact and pretreated Spirulina maxima biomass. Chemosphere 2005, 58, 125–130. [Google Scholar] [CrossRef]
- Meikle, A.J.; Gadd, G.M.; Reed, R.H. Manipulation of yeast for transport studies: Critical assessment of cultural and experimental procedures. Enzym. Microb. Technol. 1990, 12, 865–872. [Google Scholar] [CrossRef]
- Fourest, E.; Roux, J.-C. Heavy metal biosorption by fungal mycelial by-products: Mechanisms and influence of pH. Appl. Microbiol. Biotechnol. 1992, 37, 399–403. [Google Scholar] [CrossRef]
- Nandi, B.; Goswami, A.; Purkait, M. Removal of cationic dyes from aqueous solutions by kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2009, 42, 583–590. [Google Scholar] [CrossRef]
- Karthikaikumar, S.; Karthikeyan, M.; Kumar, K.S. Removal of congo red dye from aqueous solution by polyaniline–montmorrillonite composite. Chem. Sci. Rev. Lett. 2014, 2, 606–614. [Google Scholar]
- Kalotra, S.; Mehta, R. Synthesis of polyaniline/clay nanocomposites by in situ polymerization and its application for the removal of Acid Green 25 dye from wastewater. Polym. Bull. 2021, 78, 2439–2463. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Buszewski, B.; Terzyk, A.P.; Namieśnik, J. Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J. Colloid Interface Sci. 2006, 304, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, Q.; Ou, L. Kinetic, isotherm, and thermodynamic studies of the adsorption of methyl orange from aqueous solution by chitosan/alumina composite. J. Chem. Eng. Data 2012, 57, 412–419. [Google Scholar] [CrossRef]
- Alkan, M.; Demirbaş, Ö.; Çelikçapa, S.; Doğan, M. Sorption of acid red 57 from aqueous solution onto sepiolite. J. Hazard. Mater. 2004, 116, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Shirsath, S.; Patil, A.; Bhanvase, B.; Sonawane, S. Ultrasonically prepared poly (acrylamide)-kaolin composite hydrogel for removal of crystal violet dye from wastewater. J. Environ. Chem. Eng. 2015, 3, 1152–1162. [Google Scholar] [CrossRef]
- Naghizadeh, A. Regeneration of carbon nanotubes exhausted with humic acid using electro-Fenton technology. Arab. J. Sci. Eng. 2016, 41, 155–161. [Google Scholar] [CrossRef]
- Raval, N.P.; Kumar, M. Geogenic arsenic removal through core–shell based functionalized nanoparticles: Groundwater in-situ treatment perspective in the post–COVID Anthropocene. J. Hazard. Mater. 2021, 402, 123466. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Shaban, M.; Sayed, M.I.; Shahien, M.G.; Abukhadra, M.R.; Ahmed, Z.M. Adsorption behavior of inorganic-and organic-modified kaolinite for Congo red dye from water, kinetic modeling, and equilibrium studies. J. Sol-Gel Sci. Technol. 2018, 87, 427–441. [Google Scholar] [CrossRef]
- Parasuraman, D.; Sarker, A.K.; Serpe, M.J. Poly (N-Isopropylacrylamide)-Based Microgels and Their Assemblies for Organic-Molecule Removal from Water. ChemPhysChem 2012, 13, 2507–2515. [Google Scholar] [CrossRef]
- El-Aal, S.E.A.; Hegazy, E.S.A.; AbuTaleb, M.; Dessouki, A. Radiation synthesis of copolymers for adsorption of dyes from their industrial wastes. J. Appl. Polym. Sci. 2005, 96, 753–763. [Google Scholar] [CrossRef]
- Bhaumik, M.; McCrindle, R.I.; Maity, A. Enhanced adsorptive degradation of Congo red in aqueous solutions using polyaniline/Fe0 composite nanofibers. Chem. Eng. J. 2015, 260, 716–729. [Google Scholar] [CrossRef]
- Valderrama, C.; Cortina, J.; Farran, A.; Gamisans, X.; de Las Heras, F. Kinetic study of acid red “dye” removal by activated carbon and hyper-cross-linked polymeric sorbents Macronet Hypersol MN200 and MN300. React. Funct. Polym. 2008, 68, 718–731. [Google Scholar] [CrossRef]








| Series | Dye Concentration, ppm (mg/L) | Gl and Gl/PAN Weight, g | Temperature, °C | pH Value | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 10 | 15 | 20 | 25 | 0.02 | 25 | 7 | |||||||||||||
| 2 | 5 | 0.02 | 0.04 | 0.06 | 0.08 | 0.1 | 25 | 7 | |||||||||||||
| 3 | 5 | 0.02 | 25 | 40 | 50 | 60 | 7 | ||||||||||||||
| 4 | 5 | 0.02 | 25 | 2 | 3 | 4 | 6 | 7 | 8 | 12 | |||||||||||
| Langmuir isotherm | ||||||
| Constant | Qo (mg/g) | KL (L/mg) | Sd(yE±) | RL | R2 | |
| Adsorbent | ||||||
| Gl | 11.90 | 0.3300 | 0.46235 | 0.9954 | 0.9908 | |
| Gl/PAN | 14.09 | 0.4582 | 0.34417 | 0.9910 | 0.9822 | |
| Freundlich isotherm | ||||||
| constant | n | Kf | Sd(yE±) | R2 | ||
| Adsorbent | ||||||
| Gl | 2.60 | 3.6886 | 0.16929 | 0.9877 | ||
| Gl/PAN | 2.65 | 4.9243 | 0.18817 | 0.9598 | ||
| Tempkin isotherm | ||||||
| constant | B(J/mole) | KT(L/mole) | Sd(yE±) | R2 | ||
| Adsorbent | ||||||
| Gl | 2.4644 | 3.8018 | 2.51557 | 0.9790 | ||
| Gl/PAN | 2.8336 | 5.7621 | 3.29188 | 0.9417 | ||
| Catalyst | Conc, ppm | First Order | Second Order | Elovich Kinetic Model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qeexp | qe calc. | k1 | R2 | Sd(yE±) | qeexp | qe calc. | k2 | R2 | Sd(yE±) | β (g/mg) | α (mg/min) | R2 | Sd(yE±) | ||
| Gl | 25 ppm | 10 | 2.84 | 0.0044 | 0.43 | 0.48473 | 10 | 10.47 | 0.0041 | 0.99 | 0.52443 | 0.5840 | 1.6662 | 0.97 | 3.2973 |
| 20 ppm | 9.28 | 3.53 | 0.0039 | 0.66 | 0.33911 | 9.28 | 9.90 | 0.0032 | 0.98 | - | 0.6236 | 1.3069 | 0.97 | 3.08573 | |
| 15 ppm | 8.21 | 4.06 | 0.0066 | 0.28 | 0.90031 | 8.21 | 8.91 | 0.0027 | 0.97 | 1.96346 | 0.7107 | 1.0020 | 0.96 | 2.71908 | |
| 10 ppm | 6.04 | 3.02 | 0.0052 | 0.29 | 0.69973 | 6.04 | 6.59 | 0.0032 | 0.97 | 1.8975 | 0.9741 | 0.6906 | 0.95 | 1.99781 | |
| 5 ppm | 3.86 | 1.82 | 0.0038 | 0.24 | 0.56167 | 3.86 | 4.35 | 0.0039 | 0.96 | 0.37251 | 1.4881 | 0.3752 | 0.91 | 1.3324 | |
| Gl/PAN | 25 ppm | 12 | 4.95 | 0.0057 | 0.30 | 0.52443 | 12 | 12.81 | 0.0025 | 0.9915 | 11.7526 | −1.829 | −0.01189 | 0.98 | 4.01386 |
| 20 ppm | 11.57 | 2.71 | 0.0031 | 0.71 | 0.74402 | 11.57 | 15.33 | 0.002 | 0.99 | 9.81937 | −1.9608 | −0.00383 | 0.98 | 4.77362 | |
| 15 ppm | 10.07 | 61.74 | 0.0269 | 0.70 | 1.96346 | 10.07 | 10.53 | 0.00278 | 0.98 | 14.3475 | 0.59573 | 1.3501 | 0.98 | 3.21219 | |
| 10 ppm | 6.889 | 33.70 | 0.0262 | 0.71 | 1.8975 | 6.889 | 7.16 | 0.00497 | 0.99 | 21.01776 | 0.86258 | 1.0150 | 0.99 | 2.21156 | |
| 5 ppm | 4.3 | 1.68 | 0.0028 | 0.30 | 0.37251 | 4.3 | 4.58 | 0.0056 | 0.98 | 32.96873 | 1.3752 | 0.5363 | 0.98 | 1.39569 | |
| Catalyst | Conc, ppm | Intraparticle Diffusion Kinetic Model | ||
|---|---|---|---|---|
| I | k3 (mg/g min1/2) | R2 | ||
| Gl | 25 | 2.2523 | 0.4441 | 0.8361 |
| 20 | 1.6566 | 0.4297 | 0.8936 | |
| 15 | 1.1161 | 0.3891 | 0.9440 | |
| 10 | 0.7108 | 0.2876 | 0.9550 | |
| 5 | 0.2779 | 0.1933 | 0.9707 | |
| Gl/PAN | 25 | 1.9956 | 0.5646 | 0.9118 |
| 20 | 2.5729 | 0.6684 | 0.9036 | |
| 15 | 1.667 | 0.4535 | 0.9188 | |
| 10 | 1.3227 | 0.3072 | 0.8895 | |
| 5 | 0.6206 | 0.1996 | 0.9427 | |
| Adsorbent | Conditions | Reference | ||||||
|---|---|---|---|---|---|---|---|---|
| dye | Co (mg/L) | dose (g/L) | pH | Time (min) | Qm (mg/g) | R% | ||
| Modified glauconite with thermal activation | RY160 | 10–80 | 1 | 1 | 180 | - | 64% | [66] |
| Modified glauconite with acetic acid activation | RY160 | 10–80 | 1 | 1 | 180 | - | 81% | [66] |
| Phosphate-modified kaolinite | CR | 25–300 | 0.1 | 3–8 | 5–600 | - | 65% | [87] |
| Poly(N-isopropyl acrylamide-co-acrylic acid) microgel assemblies | Orange 2 | - | - | - | - | - | 73% | [88] |
| Poly(N-vinyl-2-pyrrolidone-co-acrylonitrile) treated with hydroxylamine–hydrochloride | Acid-fast Yellow G | - | - | - | - | 7.6 | - | [89] |
| PANI Nano fibers (PANI NFs) | CR | - | 1 | 7 | 30 | - | 60% | [90] |
| Poly(N-vinyl-2-pyrrolidone-co-acrylonitrile) treated with hydroxylamine–hydrochloride | Direct Blue 3B | - | - | - | - | 7 | - | [91] |
| Poly(N-vinyl-2-pyrrolidone-co-acrylonitrile) treated with hydroxylamine–hydrochloride | Reactive red SH | - | - | - | - | 7.4 | - | [89] |
| Gl Gl/PAN composite | CR CR | 5–25 5–25 | 0.02 0.02 | 7 7 | 420 420 | 11.9 14.1 | 77% 86% | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salah, D.; Hamd, A.; Soliman, N.K.; Elzanaty, A.M.; Alanazi, A.M.; Shaban, M.; El-Sayed, R.; Ahmed, S.A. Polyaniline/Glauconite Nanocomposite Adsorbent for Congo Red Dye from Textile Wastewater. Separations 2022, 9, 384. https://doi.org/10.3390/separations9110384
Salah D, Hamd A, Soliman NK, Elzanaty AM, Alanazi AM, Shaban M, El-Sayed R, Ahmed SA. Polyaniline/Glauconite Nanocomposite Adsorbent for Congo Red Dye from Textile Wastewater. Separations. 2022; 9(11):384. https://doi.org/10.3390/separations9110384
Chicago/Turabian StyleSalah, Doaa, Ahmed Hamd, N. K. Soliman, Ali M. Elzanaty, Abdulaziz M. Alanazi, Mohamed Shaban, Refat El-Sayed, and Sayed A. Ahmed. 2022. "Polyaniline/Glauconite Nanocomposite Adsorbent for Congo Red Dye from Textile Wastewater" Separations 9, no. 11: 384. https://doi.org/10.3390/separations9110384
APA StyleSalah, D., Hamd, A., Soliman, N. K., Elzanaty, A. M., Alanazi, A. M., Shaban, M., El-Sayed, R., & Ahmed, S. A. (2022). Polyaniline/Glauconite Nanocomposite Adsorbent for Congo Red Dye from Textile Wastewater. Separations, 9(11), 384. https://doi.org/10.3390/separations9110384