Parameter Effects and Optimisation in Supercritical Fluid Extraction of Phenolic Compounds from Labisia pumila
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
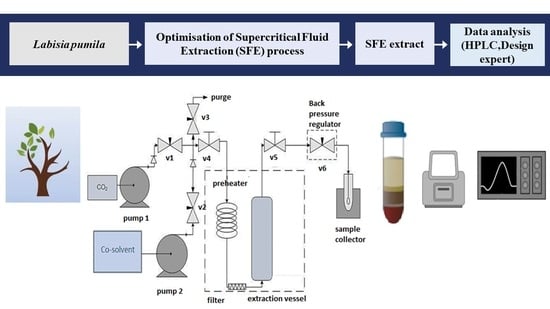
2.2. Supercritical Fluid Extraction (SFE)
2.3. Component Analysis
2.4. Experimental Design Using Response Surface Methodology (RSM) and Statistical Analysis
3. Results and Discussion
3.1. RSM Statistical Analysis
3.2. Effect of Operating Pressure on Extraction Yield and Phenolic Contents
3.3. Effect of Operating Temperature on Extraction Yield and Phenolic Contents
3.4. Effect of Ethanol Content in Co-Solvent on Extraction Yield and Phenolic Contents
3.5. Co-Solvent Concentration Effects on Extraction Yield and Phenolic Contents
3.6. Determination and Validation of Optimum Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sunamo, B. Revision of the genus Labisia (myrsinaaceae). Blumea 2005, 50, 579–597. [Google Scholar]
- Vijayalakshmi, R.; Ravindhran, R. Comparative fingerprint and extraction yield of Diospyrus ferrea (willd.) Bakh. root with phenol compounds (gallic acid), as determined by uv–vis and ft–ir spectroscopy. Asian Pac. J. Trop. Biomed. 2012, 2, S1367–S1371. [Google Scholar] [CrossRef]
- Chua, L.S.; Latiff, N.A.; Lee, S.Y.; Lee, C.T.; Sarmidi, M.R.; Aziz, R.A. Flavonoids and phenolic acids from Labisia pumila (Kacip Fatimah). Food Chem. 2011, 127, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Chermahini, S.H.; Suan, C.L.; Sarmidi, M.R. Labisia pumila: A review on its traditional, phytochemical and biological uses. World Appl. Sci. J. 2013, 10, 1297–1306. [Google Scholar]
- Karimi, E.; Jaafar, H.Z.E.; Ahmad, S. Phytochemical analysis and antimicrobial activities of methanolic extracts of leaf, stem and root from different varieties of Labisa pumila Benth. Molecules 2011, 16, 4438–4450. [Google Scholar] [CrossRef] [PubMed]
- Mohd Nazrul Hisham, D.; Mohd Lip, J.; Mohd Noh, J.; Normah, A.; Nurul Nabilah, M.F. Identification and isolation of methyl gallate as a polar chemical marker for Labisia pumila Benth. J. Trop. Agric. 2011, 39, 279–284. [Google Scholar]
- Tsao, R.; Deng, Z. Separation procedures for naturally occurring antioxidant phytochemicals. J. Chromatogr. 2004, 812, 85–99. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and anti-oxidant activity of grape marc phenolics. J Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Mohammad Azmin, S.N.H.; Abdul Manan, Z.; Wan Alwi, S.R.; Chua, L.S.; Mustaffa, A.A.; Yunus, N.A. Herbal Processing and Extraction Technologies. Sep. Purif. Rev. 2016, 45, 305–320. [Google Scholar] [CrossRef]
- Glisic, S.B.; Ristic, M.; Skala, D.U. The combined extraction of sage (Salvia officinalis L.) ultrasound followed by supercritical CO2 extraction. Ultrason Sonochem. 2011, 18, 318–326. [Google Scholar] [CrossRef]
- Hossain, M.B.; Brunton, N.P.; Patras, A.; Tiwari, B.; O’Donnell, C.P.; Martin-Diana, A.B.; Barry-Ryan, C. Optimization of ultrasound assisted extraction of antioxidant compounds from marjoram (Origanum majorana L.) using response surface methodology. Ultrason Sonochem. 2012, 19, 582–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caxambu, S.; Biondo, E.; Kolchinski, E.M.; Padilha, R.L.; Brandelli, A.; Sant’Anna, V. Evaluation of the antimicrobial ac-tivity of pecan nut [Carya illinoinensis (Wangenh) C. Koch] shell aqueous extract on minimally processed lettuce leaves. Food Sci. Technol. 2016, 36, 42–45. [Google Scholar] [CrossRef] [Green Version]
- Markom, M.; Hasan, M.; Daud, W.R.W.; Singh, H.; Jahim, J.M. Extraction of hydrolysable tannins from Phyllanthus niruri Linn.: Effects of solvents and extraction methods. Sep. Purif. Technol. 2007, 52, 487–496. [Google Scholar] [CrossRef]
- Kamarudin, N.A.; Markom, M.; Latip, J. Effects of solvents and extraction methods on herbal plants Phyllanthus niruri, Orthosiphon stamineus and Labisa pumila. J. Teknol. (Sci. Eng.) 2015, 72, 1–6. [Google Scholar]
- Hassim, N.; Markom, M.; Rosli, M.I.; Harun, S. Scale-up criteria and economic analysis for supercritical fluid extraction of Phyllanthus niruri. Chem. Eng. Process. Process Intensif. 2019, 139, 14–22. [Google Scholar] [CrossRef]
- Hassim, N.; Markom, M.; Rosli, M.I.; Harun, S. Effect of static extraction time on supercritical fluid extraction of bioactive compounds from Phyllanthus niruri. J. Comput. Theor. Nanosci. 2020, 17, 918–924. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A. Supercritical fluids extraction of polyphenols from grape seed (Vitis vinifera): Study on process variables and kinetics. J. Supercrit. Fluids 2017, 130, 239–245. [Google Scholar] [CrossRef]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2017, 23, 173–182. [Google Scholar] [CrossRef]
- Al Jitan, S.; Alkhoori, S.A.; Yousef, L.F. Studies in Natural Products Chemistry. Phenolic Acids from Plants: Extraction and Application to Human Health; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 58, pp. 389–417. [Google Scholar]
- Radzali, S.A.; Markom, M.; Baharin, B.S.; Othman, R.; Rahman, R.A. Co-solvent Selection for Supercritical Fluid Extraction of Astaxanthin and Other Carotenoids from Penaeus monodon Waste. J. Oleo Sci. 2014, 63, 763–777. [Google Scholar] [CrossRef] [Green Version]
- Mohd Sarmin, N.; Radzali, S.A.; Markom, M. Optimisation of Microencapsulation of Citrus Hystrix, L. Oil Sub and Super-critical Co2 Using Response Surface Methodology. J. Teknol. 2017, 79, 29–40. [Google Scholar]
- Markom, M.; Hassim, N.; Anuar, N.; Baharum, S.N. Co-solvent Selection for Supercritical Fluid Extraction of Essential Oil and Bioactive Compounds from Polygonum minus. ASEAN J. Chem. Eng. 2012, 12, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Reverchon, E.; Donsi, G.; Osseo, L.S. Modeling of supercritical fluid extraction from herbaceous matrices. Ind. Eng. Chem. Res. 1993, 32, 2721–2726. [Google Scholar] [CrossRef]
- Liza, M.S.; Abdul Rahman, R.; Mandana, B.; Jinap, S.; Rahmat, A.; Zaidul, I.S.M. Supercritical carbon dioxide extraction of bioactive flavonoid from Strobilanthes crispus (Pecah Kaca). Food Bioprod. Process. 2009, 88, 319–326. [Google Scholar] [CrossRef]
- Cacace, J.E.; Mazza, G. Mass transfer process during extraction of phenolic compounds from milled berries. Int. J. Food Eng. 2003, 59, 379–389. [Google Scholar] [CrossRef]
- Ng, L.Y.; Ang, Y.K.; Khoo, H.E.; Yim, H.S. Influence of different extraction parameters on antioxidant properties of Carica papaya peel and seed. Res. J. Phytochem. 2012, 6, 61–74. [Google Scholar]
- Zarena, A.S. Characterization of Bioactive Compounds from Garcinia mangostana L. Obtained by Supercritical Fluid Carbon Dioxide Process. Ph.D. Thesis, University of Mysore, Karnataka, India, 2011. [Google Scholar]
- Manohar, B.; Udaya Sankar, K. Enrichment of bakuchiol in supercritical carbon dioxide extracts of chiba seed (Psoralea corylifolia L.) using molecular distillation-Response surface methodology. Biotechnol. Bioproc. E 2009, 14, 112–117. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Radzali, S.A.; Markom, M.; Saleh, N.M. Co-Solvent Selection for Supercritical Fluid Extraction (SFE) of Phenolic Compounds from Labisia pumila. Molecules 2020, 25, 5859. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Konkol, M.; Rój, E. The Application of Supercritical Fluid Extraction in Phenolic Compounds Isolation from Natural Plant Materials. Molecules 2018, 23, 2625. [Google Scholar] [CrossRef] [Green Version]
- Colegate, S.M.; Molyneux, R.J. An introduction and overview. In Bioactive Natural Products: Detection Isolation and Structural Determination, 2nd ed.; Colegate, S.M., Molyneux, R.J., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–9. [Google Scholar]
- Lang, Q.; Wai, C.M. Supercritical fluid extraction in herbal and natural product studies—A practical review. Talanta 2001, 53, 771–782. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B.; Manikandan, S. Modeling and optimization of supercritical fluids extraction of anthocyanin and phenolic compounds from Syzygium cumini fruit pulp. J. Food Sci. Technol. 2014, 51, 1938–1946. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K.N.; Yang, B.; Shi, J.; Yu, C.; Zhao, M.; Xue, S. Enhanced antioxidant and antityrosinase activities of longan fruit pericarp by ultrahigh pressure assisted extraction. J. Pharm. Biomed. 2010, 51, 471–477. [Google Scholar] [CrossRef]
- Sonsuzer, S.; Sahin, S.; Yilmar, L. Optimization of supercritical CO2 extraction of Thymbra spicata oil. J. Supercrit. Fluids 2004, 30, 189–199. [Google Scholar] [CrossRef]
- Radzali, S.A.; Baharin, B.S.; Othman, R.; Markom, M.; Abdul Rahman, R. Optimisation of supercritical fluid extraction of astaxanthin from Penaeus Monodon waste using ethanol-modified carbon dioxide. J. Eng. Sci. Technol. 2016, 11, 722–736. [Google Scholar]
- Kryževičiute, N.; Kraujalis, P.; Venskutonis, P.R. Optimization of high pressure extraction processes for the separation of raspberry pomace into lipophilic and hydrophilic fractions. J. Supercrit. Fluids 2016, 108, 61–68. [Google Scholar] [CrossRef]
- Krichnavaruk, S.; Shotipruk, A.; Goto, M.; Pavasant, P. Supercritical carbon dioxide extraction of astaxanthin from Haem-atococcus pluvialis with vegetable oils as co-solvent. Bioresour. Technol. 2008, 99, 5556–5560. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Mantell, C.; Rodríquez, M.; Martínez de la Ossa, E.; Lubián, L.M.; Montero, O. Supercritical fluid extraction of carotenoids and chlorophyll a from Sunechococcus sp. J. Supercrit. Fluids 2007, 39, 323–329. [Google Scholar] [CrossRef]
- Brunner, G. Gas Extraction; Springer: New York, NY, USA, 1994; p. 69. [Google Scholar]
- Murga, R.; Sanz, M.T.; Beltrán, S.; Cabezas, J.L. Solubility of three hydroxycinnamic acids in supercritical carbon dioxide. J. Supercrit. Fluids 2003, 27, 239–245. [Google Scholar] [CrossRef]
- Al-Rawi, S.; Ibrahim, A.; Rahman, N.; Nama, M.; Majid, A.; Kadir, M. the effect of supercritical fluid extraction parameters on the nutmeg oil extraction and its cytotoxic and antiangiogenic properties. Procedia Food Sci. 2012, 1, 1946–1952. [Google Scholar] [CrossRef] [Green Version]
- Hawthorne, S.B.; Riekkola, M.; Serenius, K.; Holm, Y.; Hiltunen, R.; Hartonen, K. Comparison of hydrodistillation and supercritical fluid extraction for the determination of essential oils in aromatic plants. J. Chromatogr. A 1993, 634, 297–308. [Google Scholar] [CrossRef]
- Nobre, B.; Marcelo, F.; Passos, R.; Beirao, L.; Palavra, A.; Gouveia, L.; Mendes, R. Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 2006, 223, 787–790. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Hismath, I.; Wan Aida, W.M.; Ho, C.W. Optimization of extraction conditions for phenolic compounds from neem (Azadirachtaindica) leaves. Int. Food Res. J. 2011, 18, 931–939. [Google Scholar]
- Liu, C.M.; Zhao, J.M.; Li, H.M.; Song, F.R. Supercritical fluids extraction of total flavonoids from leaves of Acanthopanax Senticosus harms. Chem. Res. Chin. Univ. 2007, 23, 233–236. [Google Scholar] [CrossRef]
- Bleve, M.; Ciurlia, L.; Erroi, E.; Lionetto, G.; Longo, L.; Rescio, L.; Schettino, T.; Vasapollo, G. An innovative method for the purification of anthocyanins from grape skin extracts by using liquid and sub-critical carbon dioxide. Sep. Purific. Technol. 2008, 64, 192–197. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Marszałek, K.; Skąpska, S.; Jędrzejczak, R. The application of supercritical carbon dioxide and ethanol for the extraction of phenolic compounds from choke pomace. Appl. Sci. 2017, 7, 322. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, M.V.; Cabred, S.; Ramirez, C.L.; Fanovich, M.A. Valorization on an Agricoindustrial soybean residue by supercritical fluids extraction of phytochemical compounds. J. Supercrit. Fluids. 2019, 143, 90–96. [Google Scholar] [CrossRef]
- Farias-Campomanes, A.M.; Rostagno, M.A.; Meireles, M.A.A. Production of polyphenol extracts from grape bagasse using supercritical fluid: Yield, extract composition and economic evaluation. J. Supercrit. Fluids 2013, 77, 70–78. [Google Scholar] [CrossRef]
- Yulianthi, N.N.S.; Suhendra, l.; Wrasiati, L.P. Pengaruh perbandingan jenis pelarut terhadap kandungan senyawa total fenol, α-tokoferol, dan total karotenoid ekstrak Sargassum polycystum. J. Rekayasa Dan Manaj. Agroindustri 2017, 5, 1–10. [Google Scholar]
- Tabaraki, R.; Nateghi, A. Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ultrason. Sonochem. 2011, 18, 1279–1286. [Google Scholar] [CrossRef]
- Marsili, R.; Callahan, D. Comparison of a liquid solvent extraction technique and supercritical fluid extraction for the determination of α-and β-carotene in vegetables. J. Chromatogr. Sci. 1993, 31, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Von Rohr, P.R. High Pressure Chemistry Engineering; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Ziqiang, Z. Supercritical Fluid Technology; Chemical Industry Press: Beijing, China, 2000. [Google Scholar]
- Kinetics of Microbial Inactivation for Alternative Food Processing Technologies, High Pressure Processing. A report of the Institute of Food Technologist for the Food and Drug Administration of the U.S. Department of Health and Human Services. Available online: https://www.fda.gov/files/food/published/Evaluation-and-Definition-of-Potentially-Hazardous-Foods.pdf (accessed on 7 January 2022).
- Bennett, P.B. High Pressure Biology and Medicine; University of Rochester Press: New York, NY, USA, 1998. [Google Scholar]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effect of extraction solvents on concentration and antioxidant activity of black and black mate polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chem. 2006, 99, 838–841. [Google Scholar] [CrossRef]
- Lapornik, B.; Prosek, M.; Wondra, A.G. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Dalmolin, I.; Mazutti, M.A.; Batista, E.A.C.; Meireles, M.A.A.; Oliveira, J.V. Chemical characterization and phase behaviour of grape seed oil in compressed carbon dioxide and ethanol as co-solvent. J. Chem. Thermodyn. 2010, 42, 797–801. [Google Scholar] [CrossRef]
- Li, H.-Z.; Zhang, Z.-J.; Xue, J.; Cui, L.-X.; Hou, T.-Y.; Li, X.-J.; Chen, T. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants and rosmarinic acid from perilla leaves using response surface methodology. Food Sci. Technol. 2016, 36, 686–693. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.H.; Belanger, J.M.R.; Pare, J.R.J. Optimization of microwave-assisted extraction (MAP) for ginseng components by response surface methodology. J. Agric. Food Chem. 2003, 51, 1807–1810. [Google Scholar] [CrossRef]
- Bjorklund, E.; Jaremo, M.; Mathiasson, L.; Jonsson, J.A. Karlsson, L. Illustration of important mechanisms controlling mass transfer in supercritical fluid extraction. Anal. Chim. Acta 1998, 368, 117–128. [Google Scholar] [CrossRef]
- Murga, R.; Ruiz, R.; Beltran, S.; Cabezas, J.L. Extraction of natural complex phenols and tannins from grape seeds by using supercritical mixtures of carbon dioxide and alcohol. J. Agric. Food Chem. 2000, 48, 3408–3412. [Google Scholar] [CrossRef]







| Run Order | Pressure (Bar) | Temperature (°C) | Percentage of Ethanol in Co-Solvent (% (v/v)) | Concentration of Co-Solvent (% (v/v)) | Extraction Yield (% g/g) | Gallic Acid Content (% g/g) | Methyl Gallate Content (% g/g) | Caffeic Acid Content (% g/g) |
|---|---|---|---|---|---|---|---|---|
| 1 | 200.0 | 27.0 | 70.0 | 10.0 | 13.01 | 0.78 | 0.30 | 1.00 |
| 2 | 200.0 | 60.0 | 70.0 | 18.3 | 12.77 | 1.04 | 0.28 | 0.47 |
| 3 | 250.0 | 40.0 | 60.0 | 5.0 | 8.03 | 0.63 | 0.14 | 0.32 |
| 4 | 250.0 | 80.0 | 80.0 | 15.0 | 15.77 | 0.51 | 0.19 | 0.67 |
| 5 | 250.0 | 80.0 | 60.0 | 5.0 | 9.15 | 0.39 | 0.16 | 0.51 |
| 6 | 250.0 | 40.0 | 80.0 | 15.0 | 12.30 | 1.04 | 0.30 | 1.47 |
| 7 | 150.0 | 40.0 | 80.0 | 15.0 | 10.46 | 1.09 | 0.19 | 0.65 |
| 8 | 200.0 | 60.0 | 70.0 | 1.8 | 2.59 | 0.28 | 0.12 | 0.46 |
| 9 | 150.0 | 80.0 | 80.0 | 15.0 | 13.32 | 0.95 | 0.09 | 0.32 |
| 10 | 200.0 | 60.0 | 86.5 | 10.0 | 7.21 | 0.71 | 0.19 | 1.07 |
| 11 * | 200.0 | 60.0 | 70.0 | 10.0 | 12.93 | 0.40 | 0.21 | 0.92 |
| 12 * | 200.0 | 60.0 | 70.0 | 10.0 | 15.80 | 0.51 | 0.20 | 0.81 |
| 13 | 150.0 | 40.0 | 80.0 | 5.0 | 6.17 | 0.51 | 0.20 | 0.50 |
| 14 | 250.0 | 40.0 | 80.0 | 5.0 | 5.93 | 0.60 | 0.18 | 1.40 |
| 15 | 150.0 | 40.0 | 60.0 | 5.0 | 14.04 | 0.40 | 0.17 | 0.06 |
| 16 | 282.5 | 60.0 | 70.0 | 10.0 | 17.85 | 0.60 | 0.27 | 0.75 |
| 17 * | 200.0 | 60.0 | 70.0 | 10.0 | 14.05 | 0.55 | 0.21 | 0.79 |
| 18 | 250.0 | 80.0 | 80.0 | 5.0 | 3.97 | 0.09 | 0.14 | 0.63 |
| 19 | 117.5 | 60.0 | 70.0 | 10.0 | 13.77 | 0.48 | 0.16 | 0.42 |
| 20 | 250.0 | 40.0 | 60.0 | 15.0 | 14.96 | 1.01 | 0.47 | 0.53 |
| 21 | 250.0 | 80.0 | 60.0 | 15.0 | 20.00 | 0.71 | 0.41 | 0.48 |
| 22 | 150.0 | 40.0 | 60.0 | 15.0 | 13.50 | 0.93 | 0.31 | 0.47 |
| 23 * | 200.0 | 60.0 | 70.0 | 10.0 | 14.02 | 0.58 | 0.24 | 0.82 |
| 24 | 150.0 | 80.0 | 80.0 | 5.0 | 5.46 | 0.51 | 0.15 | 0.25 |
| 25 | 150.0 | 80.0 | 60.0 | 5.0 | 13.20 | 0.41 | 0.18 | 0.48 |
| 26 * | 200.0 | 60.0 | 70.0 | 10.0 | 14.68 | 0.42 | 0.20 | 0.84 |
| 27 * | 200.0 | 60.0 | 70.0 | 10.0 | 14.58 | 0.51 | 0.25 | 0.91 |
| 28 | 200.0 | 93.0 | 70.0 | 10.0 | 17.00 | 0.45 | 0.19 | 0.43 |
| 29 | 150.0 | 80.0 | 60.0 | 15.0 | 16.10 | 0.68 | 0.25 | 0.55 |
| 30 | 200.0 | 60.0 | 53.5 | 10.0 | 16.60 | 0.59 | 0.29 | 0.68 |
| Regression Coefficients | Extraction Yield (Y1) | Gallic Acid Content (Y2) | Methyl Gallate Content (Y3) | Caffeic Acid Content (Y4) |
|---|---|---|---|---|
| b0 | 14.420 | +0.500 | +0.221 | +0.8470 |
| b1 | +0.214 | −0.010 | +0.030 | +0.1529 |
| b2 | +0.847 | −0.121 | −0.027 | −0.1146 |
| b3 | −2.380 | +0.011 | −0.038 | +0.1457 |
| b4 | +3.140 | +0.220 | +0.054 | +0.0463 |
| b12 | +0.234 | −0.080 | −0.0008 | −0.0855 |
| b13 | +0.454 | −0.080 | −0.0062 | +0.1364 |
| b14 | +1.340 | −0.010 | +0.0377 | −0.0263 |
| b23 | −0.267 | −0.018 | −0.0122 | −0.1743 |
| b24 | +1.020 | −0.037 | −0.0183 | −0.0438 |
| b12 | +0.637 | +0.020 | - | −0.0217 |
| b21 | +0.438 | +0.013 | - | −0.0939 |
| b32 | +0.143 | +0.040 | - | −0.0463 |
| b42 | −0.995 | +0.053 | - | +0.0114 |
| R2 | 0.9585 | 0.9584 | 0.9584 | 0.9577 |
| R2 (adj) | 0.9197 | 0.9196 | 0.9196 | 0.9181 |
| Regression (p value) | <0.0001 b | <0.0001 b | <0.0001 b | <0.0001 b |
| lack of fit (p value) | 0.2224 c | 0.5666 c | 0.5666 c | 0.0721 c |
| Variables | Extraction Yield (Y1) | Gallic Acid Content (Y2) | Methyl Gallate Content (Y3) | Caffeic Acid Content (Y4) | |||||
|---|---|---|---|---|---|---|---|---|---|
| F Ratio | p Value | F Ratio | p Value | F Ratio | p Value | F Ratio | p Value | ||
| Main effects | X1 | 0.64 | 0.4349 | 0.43 | 0.5240 | 52.51 | <0.0001 | 62.20 | <0.0001 |
| X2 | 10.11 | 0.0062 | 63.48 | <0.0001 | 42.32 | <0.0001 | 34.97 | <0.0001 | |
| X3 | 80.08 | <0.0001 | 0.56 | 0.4668 | 84.86 | <0.0001 | 56.51 | <0.0001 | |
| X4 | 138.83 | <0.0001 | 208.01 | <0.0001 | 176.41 | <0.0001 | 5.71 | 0.0305 | |
| Quadratic effects | X12 | 1.95 | 0.1834 | 0.55 | 0.4683 | - | - | 16.86 | 0.0009 |
| X22 | 0.21 | 0.6545 | 4.85 | 0.0438 | - | - | 4.10 | 0.0610 | |
| X32 | 10.04 | 0.0064 | 8.75 | 0.0098 | - | - | 0.25 | 0.6250 | |
| X42 | 65.81 | <0.0001 | 9.77 | 0.0070 | - | - | 37.00 | <0.0001 | |
| Interaction effects | X1X2 | 0.58 | 0.4590 | 20.53 | 0.0004 | 0.025 | 0.8757 | 14.52 | 0.0017 |
| X1X3 | 2.16 | 0.1619 | 18.97 | 0.0006 | 1.72 | 0.2051 | 36.95 | <0.0001 | |
| X1X4 | 18.91 | 0.0006 | 0.30 | 0.5938 | 63.42 | <0.0001 | 1.38 | 0.2591 | |
| X2X3 | 0.75 | 0.3996 | 1.06 | 0.3186 | 6.66 | 0.0183 | 60.32 | <0.0001 | |
| X2X4 | 10.99 | 0.0047 | 4.44 | 0.0523 | 14.93 | 0.0010 | 3.81 | 0.0700 | |
| X3X4 | 4.27 | 0.0566 | 1.18 | 0.2952 | 81.95 | <0.0001 | 0.93 | 0.3491 | |
| Run | Optimum Extraction Condition Predicted | Extraction Yield % (g/g) | Gallic Acid Content (% g/g) | Methyl Gallate Content (% g/g) | Caffeic Acid Content (% g/g) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Yo | Yi | Yo | Yi | Yo | Yi | Yo | Yi | ||
| 1 | 283 bar, 32 °C, 78% (v/v), 16% (v/v) | 14.05 ± 0.76 | 14.34 | 1.27 ± 0.10 | 1.34 | 0.44 ± 0.29 | 0.42 | 1.38 ± 0.37 | 1.47 |
| 2 | 283 bar, 32 °C, 79% (v/v), 17% (v/v) | 12.74 ± 0.05 | 13.92 | 1.32 ± 0.92 | 1.40 | 0.30 ± 0.51 | 0.42 | 1.50 ± 0.05 | 1.47 |
| 3 | 240 bar, 32 °C, 75% (v/v), 18% (v/v) | 11.89 ± 0.49 | 11.48 | 1.40 ± 0.36 | 1.38 | 0.37 ± 0.18 | 0.403 | 1.08 ± 0.18 | 1.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzali, S.A.; Markom, M.; Md Saleh, N. Parameter Effects and Optimisation in Supercritical Fluid Extraction of Phenolic Compounds from Labisia pumila. Separations 2022, 9, 385. https://doi.org/10.3390/separations9120385
Radzali SA, Markom M, Md Saleh N. Parameter Effects and Optimisation in Supercritical Fluid Extraction of Phenolic Compounds from Labisia pumila. Separations. 2022; 9(12):385. https://doi.org/10.3390/separations9120385
Chicago/Turabian StyleRadzali, Shazana Azfar, Masturah Markom, and Noorashikin Md Saleh. 2022. "Parameter Effects and Optimisation in Supercritical Fluid Extraction of Phenolic Compounds from Labisia pumila" Separations 9, no. 12: 385. https://doi.org/10.3390/separations9120385
APA StyleRadzali, S. A., Markom, M., & Md Saleh, N. (2022). Parameter Effects and Optimisation in Supercritical Fluid Extraction of Phenolic Compounds from Labisia pumila. Separations, 9(12), 385. https://doi.org/10.3390/separations9120385