Removal of Patent Blue Dye Using Ananas comosus-Derived Biochar: Equilibrium, Kinetics, and Phytotoxicity Studies
Abstract
:1. Introduction
2. Materials and Methods
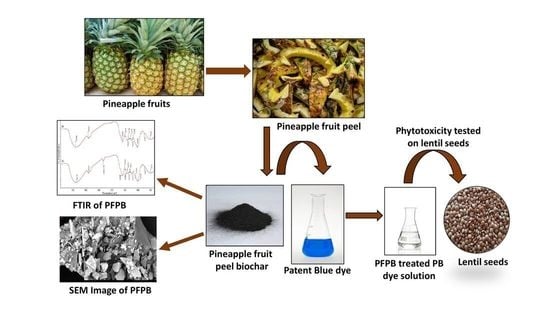
2.1. Ananas Comosus Fruit Peel Biochar
2.2. Materials and Preparation of Reagents
Instrumentations
2.3. Batch Study
2.4. Point Zero of Charge
2.5. Depiction of Pineapple Fruit Peel Biochar
2.6. Adsorption Isotherm
2.6.1. Langmuir Isotherm
2.6.2. Freundlich Isotherm
2.6.3. Temkin Isotherm
2.7. Kinetics of Adsorption Process
2.8. Thermodynamic Variables
2.9. Reusability Study
2.10. Estimation of Phytotoxicity
2.11. Estimation of Biochemical Constituents
2.12. Statistical Analysis
3. Results and Discussion
3.1. Proximate Assessment of Adsorbent
3.2. Properties of Biochar Prepared from Pineapple Fruit Peel
3.3. Effect of Different Variables on Uptake of Patent Blue Dye by Pineapple Fruit Peel Biochar
3.3.1. pH
3.3.2. Contact Time
3.3.3. Adsorbent Amount
3.3.4. Concentration of Dye
3.3.5. Agitation Speed
3.3.6. Temperature
3.4. Point of Zero Charge
3.5. Equilibrium Modelling
3.6. Adsorption Kinetics
3.7. Thermodynamic Analysis
3.8. Regeneration Analysis
3.9. Estimation of Phytotoxicity
3.10. Pineapple Fruit Peel Biochar Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golinska, P.; Belbahri, L. Diversity of synthetic dyes from textile industries, discharge impacts and treatment methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Qiu, H.; Shen, F.; Yin, A.; Liu, J.; Wu, B.; Li, Y.; Xiao, Y.; Hai, J.; Xu, B. Biodegradation and detoxification of azo dyes by halophilic/halotolerant microflora isolated from the salt fields of Tibet autonomous region China. Front. Microbiol. 2022, 13, 877151. [Google Scholar] [CrossRef]
- Teo, S.W.; Ng, C.H.; Islam, A.; Abdulkareem-Alsultan, G.; Joseph, C.G.; Janaun, J.; Taufiq-Yap, Y.H.; Khandaker, S.; Islam, G.J.; Znad, H.; et al. Sustainable toxic dyes removal with advanced materials for clean water production: A comprehensive review. J. Clean. Prod. 2022, 332, 130039. [Google Scholar] [CrossRef]
- Pattanaik, L.; Duraivadivel, P.; Hariprasad, P.; Naik, S.N. Utilization and re-use of solid and liquid waste generated from the natural indigo dye production process—A zero waste approach. Bioresour. Technol. 2020, 301, 122721. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Srinivas Kini, M.; Mythili, R.; Selvaraj, R. Adsorptive removal of AB113 dye using green synthesized hydroxyapatite/magnetite nanocomposite. Environ. Res. 2022, 210, 112951. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.; El-Shafie, A.S.; Zaher, N.; El-Azazy, M. Application of pineapple leaves as adsorbents for removal of rose bengal from wastewater: Process optimization operating face-centered central composite design. Molecules 2020, 25, 3752. [Google Scholar] [CrossRef]
- Fernandes, J.V.; Rodrigues, A.M.; Menezes, R.R.; de Araujo Neves, G. Adsorption of anionic dye on the acid-functionalized bentonite. Materials 2020, 13, 3600. [Google Scholar] [CrossRef]
- Cai, B.; Feng, J.F.; Peng, Q.Y.; Zhao, H.F.; Miao, Y.C.; Pan, H. Super-fast degradation of high concentration methyl orange over bifunctional catalyst Fe/Fe3C@C with microwave irradiation. J. Hazard. Mater. 2020, 392, 122279. [Google Scholar] [CrossRef]
- Husunet, M.T.; Misirli, R.C.; Istifli, E.S.; Basrilla, H. Investigation of the genotoxic effects of patent blue (E131) in human peripheral lymphocytes and in silico molecular docking. Drug Chem. Toxicol. 2022, 45, 1780–1786. [Google Scholar] [CrossRef]
- Tacas, A.C.J.; Tsai, P.W.; Tayo, L.L.; Hsueh, C.C.; Sun, S.Y.; Chen, B.Y. Degradation and biotoxicity of azo dyes using indigenous bacteria-acclimated microbial fuel cells (MFCs). Process. Biochem. 2021, 102, 59–71. [Google Scholar] [CrossRef]
- Albukhari, S.M.; Abdel Salam, M.; Aldawsari, A.M.M. Removal of malachite green dye fromwater using MXene (Ti3C2) nanosheets. Sustainability 2022, 14, 5996. [Google Scholar] [CrossRef]
- Harja, M.; Buema, G.; Bucur, D. Recent advances in removal of Congo Red dye by adsorption using an industrial waste. Sci. Rep. 2022, 12, 6087. [Google Scholar] [CrossRef] [PubMed]
- Partlan, E.; Ren, Y.; Apul, O.G.; Ladner, D.A.; Karanfil, T. Adsorption kinetics of synthetic organic contaminants onto superfine powdered activated carbon. Chemosphere 2020, 253, 126628. [Google Scholar] [CrossRef] [PubMed]
- Arabkhani, P.; Javadian, H.; Asfaram, A.; Sadeghfar, F.; Sadegh, F. Synthesis of magnetic tungsten disulfide/carbon nanotubes nanocomposite (WS2/Fe3O4/CNTs-NC) for highly efficient ultrasound-assisted rapid removal of amaranth and brilliant blue FCF hazardous dyes. J. Hazard. Mater. 2021, 420, 126644. [Google Scholar] [CrossRef]
- Yu, J.; Zou, J.; Xu, P.; He, Q. Three-dimensional photoelectrocatalytic degradation of the opaque dye acid fuchsin by Pr and Co co-doped TiO2 particle electrodes. J. Clean. Prod. 2020, 251, 119744. [Google Scholar] [CrossRef]
- Ismail, G.A.; Sakai, H. Review on effect of different type of dyes on advanced oxidation processes (AOPs) for textile color removal. Chemosphere 2021, 291, 132906. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Lai, H.J. Adsorption of remazol brilliant violet 5R (RBV-5R) and remazol brilliant blue R (RBBR) from aqueous solution by using agriculture waste. Trop. Aquat. Soil Pollut. 2021, 1, 11–23. [Google Scholar] [CrossRef]
- Birniwa, A.H.; Mahmud, H.N.M.E.; Abdullahi, S.S.; Habibu, S.; Jagaba, A.H.; Ibrahim, M.N.M.; Ahmad, A.; Alshammari, M.B.; Parveen, T.; Umar, K. Adsorption behavior of methylene blue cationic dye in aqueous solution using polypyrrole-polyethylenimine nano-adsorbent. Polymers 2022, 14, 3362. [Google Scholar] [CrossRef]
- Chin-Pampillo, J.S.; Alfaro-Vargas, A.; Rojas, R.; Giacomelli, C.E.; Perez-Villanueva, M.; Chinchilla-Soto, C.; Alcañiz, J.M.; Domene, X. Widespread tropical agrowastes as novel feedstocks for biochar production: Characterization and priority environmental uses. Biomass Conv. Bioref. 2021, 11, 1775–1785. [Google Scholar] [CrossRef]
- Issaka, E.; Fapohunda, F.O.; Amu-Darko, J.N.O.; Yeboah, L.; Yakubu, S.; Varjani, S.; Ali, N.; Bilal, M. Biochar-based composites for remediation of polluted wastewater and soil environments: Challenges and prospects. Chemosphere 2022, 297, 134163. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, G.; Gopinath, K.A.; Reddy, K.S.; Reddy, B.S.; Prabhakar, M.; Srinivasarao, C.; Visha Kumari, V.; Singh, V.K. Characterization of biochar derived from crop residues for soil amendment, carbon sequestration and energy use. Sustainability 2022, 14, 2295. [Google Scholar] [CrossRef]
- Brinza, L.; Maftei, A.E.; Tascu, S.; Brinza, F.; Neamtu, M. Advanced removal of Reactive Yellow 84 azo dye using functionalised amorphous calcium carbonates as adsorbent. Sci. Rep. 2022, 12, 3112. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Carr, C.M. Biomass-derived porous carbonaceous materials and their composites as adsorbents for cationic and anionic dyes: A review. Chemosphere 2021, 265, 129087. [Google Scholar] [CrossRef]
- Sackey, E.A.; Song, Y.; Yu, Y.; Zhuang, H. Biochars derived from bamboo and rice straw for sorption of basic red dyes. PLoS ONE 2021, 16, e0254637. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Rafatullah, M.; Siddiqui, M.R.; Khan, M.A.; Sillanpaa, M. Removal of reactive black 5 dye by banana peel biochar and evaluation of its phytotoxicity on tomato. Sustainability 2022, 14, 4176. [Google Scholar] [CrossRef]
- Thomas, T.; Thalla, A.K. Nutmeg seed shell biochar as an effective adsorbent for removal of remazol brilliant blue reactive dye: Kinetic, isotherm, and thermodynamic study. Energy Sources A Recovery Util. Environ. Eff. 2022, 44, 893–911. [Google Scholar] [CrossRef]
- Birniwa, A.H.; Mohammad, R.E.A.; Ali, M.; Rehman, M.F.; Abdullahi, S.S.; Eldin, S.M.; Mamman, S.; Sadiq, A.C.; Jagaba, A.H. Synthesis of gum arabic magnetic nanoparticles for adsorptive removal of ciprofloxacin: Equilibrium, kinetic, thermodynamics studies and optimization by response surface methodology. Separations 2022, 9, 322. [Google Scholar] [CrossRef]
- Uma, S.; Thalla, A.K.; Devatha, C.P. Co-digestion of food waste and switchgrass for biogas potential: Effects of process parameters. Waste Biomass Valoris. 2020, 11, 827–839. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Vieira, R.A.L.; Pickler, T.B.; Segato, T.C.M.; Jozala, A.F.; Grotto, D. Biochar from fungiculture waste for adsorption of endocrine disruptors in water. Sci. Rep. 2022, 12, 6507. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Hashim, N.; Aziz, S.A.; Lasekan, O. Pineapple (Ananas comosus): A comprehensive review of nutritional values, volatile compounds, health benefits, and potential food products. Food Res. Int. 2020, 137, 109675. [Google Scholar]
- Agnihotri, S.; Sillu, D.; Sharma, G.; Arya, R.K. Photocatalytic and antibacterial potential of silver nanoparticles derived from pineapple waste: Process optimization and modeling kinetics for dye removal. Appl. Nanosci. 2018, 8, 2077–2092. [Google Scholar] [CrossRef]
- Aili Hamzah, A.F.; Hamzah, M.H.; Che Man, H.; Jamali, N.S.; Siajam, S.I.; Ismail, M.H. Recent updates on the conversion of pineapplewaste (Ananas comosus) to value-added products, future perspectives and challenges. Agronomy 2021, 11, 2221. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Zhang, H.; Ma, L.; Huang, H.; Wu, J.; Zhang, Y. Direct fabrication of hierarchically processed pineapple peel hydrogels for efficient Congo red adsorption. Carbohydr. Polym. 2020, 230, 115599. [Google Scholar] [CrossRef] [PubMed]
- Konczyk, J.; Kluziak, K.; Kołodynska, D. Adsorption of vanadium (V) ions from the aqueous solutions on different biomass-derived biochars. J. Environ. Manag. 2022, 313, 114958. [Google Scholar] [CrossRef]
- Celebi, H. The applicability of evaluable wastes for the adsorption of reactive black 5. Int. J. Environ. Sci. Technol. 2018, 16, 135–146. [Google Scholar] [CrossRef]
- Cebrian, G.; Condon, S.; Manas, P. Physiology of the inactivation of vegetative bacteria by thermal treatments: Mode of action, influence of environmental factors and inactivation kinetics. Foods 2017, 6, 107. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-Garcia, M.A.; Moreno-Castilla, C. Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J. Chem. Technol. Biotechnol. 2001, 76, 1209–1215. [Google Scholar] [CrossRef] [Green Version]
- Bharathi, K.S.; Ramesh, S.P.T. Fixed-bed column studies on biosorption of crystal violet from aqueous solution by Citrullus lanatus rind and Cyperus rotundus. Appl. Water Sci. 2013, 3, 673–687. [Google Scholar] [CrossRef]
- Ng, J.C.Y.; Cheung, W.H.; McKay, G. Equilibrium Studies of the Sorption of Cu(II) Ions onto chitosan. J. Colloid Interface Sci. 2002, 255, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Temkin, M.I.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys. Chim. USSR 1940, 12, 327–356. [Google Scholar]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Mckay, G. Pseudo-second-order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Sivamani, S. Exploring the potential of Eucalyptus citriodora biochar against direct red 31 dye and its phytotoxicity assessment. Biomass Conv. Bioref. 2021, 24, 1–12. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; International Seed Testing Association, ISTA Secretariat: Bassersdorf, Switzerland, 2008. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Hedge, J.E.; Hofreiter, B.T. Carbohydrate Chemistry 17; Whistler, R.L., Be Miller, J.N., Eds.; Academic Press: New York, NY, USA, 1962; pp. 17–22. [Google Scholar]
- Shakya, A.; Agarwal, T. Removal of Cr(VI) from water using pineapple peel derived biochars: Adsorption potential and re-usability assessment. J. Mol. Liq. 2019, 293, 111497. [Google Scholar] [CrossRef]
- Waqas, M.; Aburiazaiza, A.S.; Miandad, R.; Rehan, M.; Barakat, M.A.; Nizami, A.S. Development of biochar as fuel and catalyst in energy recovery technologies. J. Clean. Prod. 2018, 188, 477–488. [Google Scholar] [CrossRef]
- Lee, S.L.; Park, J.H.; Kim, S.H.; Kang, S.W.; Cho, J.S.; Jeon, J.R.; Lee, Y.B.; Seo, D.C. Sorption behavior of malachite green onto pristine lignin to evaluate the possibility as a dye adsorbent by lignin. Appl. Biol. Chem. 2019, 62, 37. [Google Scholar] [CrossRef] [Green Version]
- Wisniewska, M.; Chibowski, S.; Wawrzkiewicz, M.; Onyszko, M.; Bogatyrov, V.C.I. Basic Red 46 removal from sewage by carbon and silica based composite: Equilibrium, kinetic and electrokinetic studies. Molecules 2022, 27, 1043. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Ramesh, S.T.; Gandhimathi, R.; Nidheesh, P.V. Pineapple leaf (Ananas comosus) powder as a biosorbent for the removal of crystal violet from aqueous solution. Desalin Water Treat. 2014, 54, 2041–2054. [Google Scholar] [CrossRef]
- Salameh, Y.; Albadarin, A.B.; Allen, S.; Walker, G.; Ahmad, M.N.M. Arsenic (III, V) adsorption onto charred dolomite: Charring optimization and batch studies. Chem. Eng. J. 2015, 259, 663–671. [Google Scholar] [CrossRef]
- Jamali-Behnam, F.; Najafpoor, A.A.; Davoudi, M.; Rohani-Bastami, T.; Alidadi, H.; Esmaily, H.; Dolatabadi, M. Adsorptive removal of arsenic from aqueous solutions using magnetite nanoparticles and silica-coated magnetite nanoparticles. Environ. Prog. Sustain. Energy 2018, 37, 951–960. [Google Scholar] [CrossRef]
- Dai, H.; Huang, H. Modified pineapple peel cellulose hydrogels embedded with sepia inkfor effective removal of methylene blue. Carbohydr. Polym. 2016, 148, 1–10. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Noor, A.; Isah, A.S.; Lawal, I.M.; Birniwa, A.H.; Usman, A.K.; Abubakar, S. Kinetics of pulp and paper wastewater treatment by high sludge retention time activated sludge process. J. Hunan Univ. (Nat. Sci.) 2022, 49, 242–251. [Google Scholar] [CrossRef]
- Duarte Neto, J.F.; Pereira, I.D.S.; Da Silva, V.C.; Ferreira, H.C.; Neves, D.G.A.; Menezes, R.R. Study of equilibrium and kinetic adsorption of rhodamine B onto purified bentonite clays. Ceramica 2018, 64, 598–607. [Google Scholar] [CrossRef]
- Perez-Calderon, J.; Santos, M.V.; Zaritzky, N. Reactive red 195 dye removal using chitosan coacervated particles as bio-sorbent: Analysis of kinetics, equilibrium and adsorption mechanisms. J. Environ. Chem. Eng. 2018, 6, 6749–6760. [Google Scholar] [CrossRef] [Green Version]
- Karthick, K.; Namasivayam, C.; Pragasam, L.A. Removal of direct red 12B from aqueous medium by ZnCl2 activated Jatropha husk carbon: Adsorption dynamics and equilibrium studies. Ind. J. Chem. Technol. 2017, 24, 73–81. [Google Scholar]
- Birniwa, A.H.; Abubakar, A.S.; Mahmud, H.N.M.E.; Kutty, S.R.M.; Jagaba, A.H.; Abdullahi, S.S.; Zango, Z.U. Application of agricultural wastes for cationic dyes removal from wastewater. In Textile Wastewater Treatment: Sustainable Textiles: Production, Processing, Manufacturing & Chemistry; Muthu, S.S., Khadir, A., Eds.; Springer: Singapore, 2022; pp. 239–274. [Google Scholar]
- Baloo, L.; Isa, M.H.; Sapari, N.B.; Jagaba, A.H.; Wei, L.J.; Yavari, S.; Razali, R.; Vasu, R. Adsorptive removal of methylene blue and acid orange 10 dyes from aqueous solutions using oil palm wastes-derived activated carbons. Alex. Eng. J. 2021, 60, 5611–5629. [Google Scholar] [CrossRef]
- Pavan, F.A.; Mazzocato, A.C.; Gushiken, Y. Removal of methylene blue dye from aqueous solutions by adsorption using yellow passion fruit peel as adsorbent. Bioresour. Technol. 2008, 99, 3162–3165. [Google Scholar] [CrossRef] [PubMed]
- Samarghandy, M.R.; Hoseinzade, E.; Taghavi, M.; Hoseinzadeh, S. Biosorption of reactive black 5 from aqueous solution using acid-treated biomass from potato peel waste. Bioresources 2011, 6, 4840–4855. [Google Scholar]
- Senthil Kumar, P.; Ramalingam, S.; Senthamarai, C.; Niranjanna, M.; Vijayalakshmi, P.; Sivanesan, S. Adsorption of dye from aqueous solution by cashew nut shell: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Desalination 2010, 261, 52–60. [Google Scholar] [CrossRef]









| Dye Stuff | Acid Blue 3 or Sulphan Blue or Food Blue 5 |
|---|---|
| C.I. Number | 42,051 |
| Color | Blue color solid |
| Type Melting point | Anionic dye 200 °C |
| Solubility | Water soluble |
| IUPAC name | calcium;4-[[4-(diethylamino)phenyl]-(4-diethylazaniumylidenecyclohexa-2,5-dien-1-ylidene)methyl]-6-hydroxybenzene-1,3-disulfonate |
| Formula | C54H62CaN4O14S4 |
| Molecular weight Application | 1159.4 g/mol Utilized in textiles, printing, and food industries |
| Structure |  |
| λmax | 635 nm |
| Isotherm | Equation | Parameters | Value |
|---|---|---|---|
| Langmuir | qm (mg/g) | 10.29 | |
| KL (L/mg) | 0.03 | ||
| R2 | 0.81 | ||
| Freundlich | n | 1.07 | |
| KF (mg/g) | 2.92 | ||
| R2 | 0.19 | ||
| Temkin | qe = RT/bT In(AT) + RT/bT In(Ce) | bT (J/mole) | 3.71 |
| AT (L/mole) | 1.19 | ||
| R2 | 0.17 |
| Model | Equation | Parameters | Value |
|---|---|---|---|
| Pseudo first-order | K1 (min−1) | 0.003 | |
| qe (mg/g) | 2.73 | ||
| R2 | 0.43 | ||
| Pseudo second-order | K2 (g/mg min) | 0.04 | |
| qe (mg/g) | 15.04 | ||
| R2 | 0.99 | ||
| Weber–Morris intraparticle diffusion | qt = kdiff t1/2 + C | Kdiff | 0.03 |
| C | 7.19 | ||
| R2 | 0.46 |
| S.No. | Temperature (°C) | (∆G0) (KJ mol−1) | ΔH0 (KJ mol−1) | ΔS0 (J/K) |
|---|---|---|---|---|
| 1. | 25 | −5875.19 | −34.17 | 97.15 |
| 2. | 30 | −5331.57 | ||
| 3. | 35 | −4185.83 | ||
| 4. | 40 | −1150.56 | ||
| 5. | 45 | −2739.12 | ||
| 6. | 50 | −4637.64 |
| Treatment | Germination (%) | Plumule Length | Radicle Length | Vigor Index |
|---|---|---|---|---|
| Control | 96 ± 0.71 a | 9.13 ± 0.08 a | 3.44 ± 0.10 a | 12,067.2 |
| PB dye solution (600 mg/L) | 13 ± 0.82 c | 2.31 ± 0.19 c | 0.61 ± 0.02 c | 379.6 |
| PFPB treated PB dye solution | 78 ± 0.82 b | 7.24 ± 0.17 b | 1.89 ± 0.06 b | 7304 |
| Treatment | Chlorophyll (mg/g FW) | Sugar (mg/g DW) | Protein (mg/g FW) |
|---|---|---|---|
| Control | 3.27 ± 0.11 a | 3.36 ± 0.22 a | 23.37 ± 0.58 a |
| PB dye solution (600 mg/L) | 0.62 ± 0.03 c | 1.27 ± 0.09 c | 5.25 ± 0.16 d |
| PFPB treated PB dye solution | 2.16 ± 0.08 b | 2.44 ± 0.12 b | 17.43 ± 0.15 b |
| Biochar | Dye | Experimental Conditions | qmax (mg/g) | References |
|---|---|---|---|---|
| Yellow passion fruit peel | Methylene Blue | pH = 9 | 0.01 | Pavan et al. [66] |
| Exposure time = 56 h | ||||
| Adsorbent dose = 1 g/50 mL | ||||
| Potato peel | Reactive Black5 | pH = 3 | 3.61 | Samarghandy et al. [67] |
| Exposure time = 60 min | ||||
| Adsorbent dose = 1 g/50 mL | ||||
| Cashew nut shell | Congo Red | pH = 3 | 5.18 | Senthilkumar et al. [68] |
| Exposure time = 90 min | ||||
| Adsorbent dose = 30 g/L | ||||
| Banana peel | Reactive Black 5 | pH = 3 | 7.58 | Kapoor et al. [27] |
| Exposure time = 120 min | ||||
| Adsorbent dose = 0.8 g/100 mL | ||||
| Pineapple fruit peel | Patent Blue | pH = 2 | 10.29 | This study |
| Exposure time = 240 min | ||||
| Adsorbent dose = 3.5 g/100 mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapoor, R.T.; Rafatullah, M.; Aljuwayid, A.M.; Habila, M.A.; Wabaidur, S.M.; Alam, M. Removal of Patent Blue Dye Using Ananas comosus-Derived Biochar: Equilibrium, Kinetics, and Phytotoxicity Studies. Separations 2022, 9, 426. https://doi.org/10.3390/separations9120426
Kapoor RT, Rafatullah M, Aljuwayid AM, Habila MA, Wabaidur SM, Alam M. Removal of Patent Blue Dye Using Ananas comosus-Derived Biochar: Equilibrium, Kinetics, and Phytotoxicity Studies. Separations. 2022; 9(12):426. https://doi.org/10.3390/separations9120426
Chicago/Turabian StyleKapoor, Riti Thapar, Mohd Rafatullah, Ahmed Muteb Aljuwayid, Mohamed A. Habila, Saikh Mohammad Wabaidur, and Mahboob Alam. 2022. "Removal of Patent Blue Dye Using Ananas comosus-Derived Biochar: Equilibrium, Kinetics, and Phytotoxicity Studies" Separations 9, no. 12: 426. https://doi.org/10.3390/separations9120426
APA StyleKapoor, R. T., Rafatullah, M., Aljuwayid, A. M., Habila, M. A., Wabaidur, S. M., & Alam, M. (2022). Removal of Patent Blue Dye Using Ananas comosus-Derived Biochar: Equilibrium, Kinetics, and Phytotoxicity Studies. Separations, 9(12), 426. https://doi.org/10.3390/separations9120426