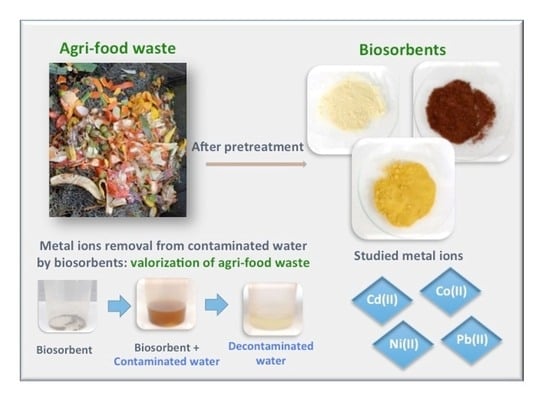
Potential Use of Low-Cost Agri-Food Waste as Biosorbents for the Removal of Cd(II), Co(II), Ni(II) and Pb(II) from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cleaning Protocol
2.2. Reagents and Solutions
2.3. Preparation of the Biosorbents
2.4. Batch Biosorption Studies
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saval, S. Aprovechamiento de residuos agroindustriales: Pasado, presente y futuro. BioTecnología 2012, 16, 14–46. [Google Scholar]
- Alslaibi, T.; Ismail, A.; Mohd, A.; Ahmad, M.A.; Foul, A.A. Heavy metals removal from wastewater using agricultural wastes as adsorbents: A review. Int. J. Chem. Environ. Eng. 2014, 5, 7–10. [Google Scholar]
- Salim, R.M.D.; Asik, J.; Sarjadi, M.S. Chemical functional groups of extractives, cellulose and lignin extracted from native Leucaena leucocephala bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Lu, Y.; He, Q.; Fan, G.; Cheng, Q.; Song, G. Extraction and modification of hemicellulose from lignocellulosic biomass: A review. Green Process. Synth. 2021, 10, 779–804. [Google Scholar] [CrossRef]
- Agrowaste, Consejo Superior de Investigaciones Científicas (CSIC). Available online: http://ctnc.es/proyectos/agrowaste-life/ (accessed on 27 July 2021).
- Agrocycle. Available online: http://www.agrocycle.eu/#project (accessed on 27 July 2021).
- Vílchez, R. Eliminación de Metales Pesados en Aguas Mediante Sistemas de Lechos Sumergidos: Estudio Microbiológico en las Biopelículas. Ph.D. Thesis, Universidad de Granada, Instituto del Agua, España, 2005. [Google Scholar]
- Nobi, E.P.; Dilipan, E.; Thangaradjou, T.; Sivakumar, K.; Kannan, L. Geochemical and geostatistical assessment of heavy metal concentration in the sediments of different coastal ecosystems of Andaman Islands, India. Estuar. Coast. Shelf Sci. 2010, 87, 253–264. [Google Scholar] [CrossRef]
- Soriano Francia, J.H. Saneamiento de Suelos por Fitorremediación de los Cultivos de Plátanos en el Fundo San José del Distrito de Mala; Informe Final de Proyecto de Investigación; Universidad Nacional del Callao, Facultad de Ingeniería Química, Instituto de Investigación de la Facultad de Ingeniería Química: Bellavista, Perú, 2015. [Google Scholar]
- Arslanoglu, H.; Altundogan, H.S.; Tumen, F. Preparation of cation exchanger from lemon and sorption of divalent heavy metals. Bioresour. Technol. 2008, 99, 2699–2705. [Google Scholar] [CrossRef] [Green Version]
- Tejada-Tovar, C.; Villabona-Ortiz, A.; Garcés-Jaraba, L. Adsorción de metales pesados en aguas residuales usando materiales de origen biológico. TecnoLógicas 2015, 18, 109–123. [Google Scholar] [CrossRef] [Green Version]
- Villanueva Huerta, C.C. Biosorción de Cobre (II) por Biomasa Pretratada de Cáscara de Citrus sinensis (Naranja), Citrus limonium (Limón) y Opuntia ficus (Palmeta de Nopal). Bachelor’s Thesis, Universidad Nacional Mayor de San Marcos, Facultad de Química e Ingeniería Química, Lima, Perú, 2007. [Google Scholar]
- Chojnacka, K.; Mikulewicz, M. Green analytical methods of metals determination in biosorption studies. TrAC Trends Anal. Chem. 2019, 116, 254–265. [Google Scholar] [CrossRef]
- Sathish, S.; Prasad, B.S.N.; Kumar, J.A.; Prabu, D.; Sivamani, S. Batch and column studies for adsorption of naphthalene from its aqueous solution using nanochitosan/sodium alginate composite. Polym. Bull. 2022, 79, 8695–8715. [Google Scholar] [CrossRef]
- Kumar, J.A.; Amarnath, D.J.; Sathish, S.; Jabasingh, S.A.; Saravanan, A.; Hemavathy, R.V.; Anand, K.V.; Yaashikaa, P.R. Enhanced PAHs removal using pyrolysis-assisted potassium hydroxide induced palm shell activated carbon: Batch and column investigation. J. Mol. Liq. 2019, 279, 77–87. [Google Scholar] [CrossRef]
- Kumar, J.A.; Kumar, P.S.; Krithiga, T.; Prabu, D.; Amarnath, D.J.; Sathish, S.; Venkatesan, D.; Hosseini-Bandegharaei, A.; Prashant, P. Acenaphthene adsorption onto ultrasonic assisted fatty acid mediated porous activated carbon-characterization, isotherm and kinetic studies. Chemosphere 2021, 284, 131249. [Google Scholar] [CrossRef]
- Kumar, J.A.; Krithiga, T.; Anand, K.V.; Sathish, S.; Namasivayam, S.K.R.; Renita, A.A.; Hosseini-Bandeghar, A.; Praveenkumar, T.R.; Rajasimman, M.; Bhat, N.S.; et al. Kinetics and regression analysis of phenanthrene adsorption on the nanocomposite of CaO and activated carbon: Characterization, regeneration, and mechanistic approach. J. Mol. Liq. 2021, 334, 116080. [Google Scholar] [CrossRef]
- Prabu, D.; Kumar, P.S.; Rathi, B.S.; Sathish, S.; Anand, K.V.; Kumar, J.A.; Mohammed, O.B.; Silambarasan, P. Feasibility of magnetic nano adsorbent impregnated with activated carbon from animal bone waste: Application for the chromium (VI) removal. Environ. Res. 2022, 203, 111813. [Google Scholar] [CrossRef]
- Kumar, J.A.; Krithiga, T.; Narendrakumar, G.; Prakash, P.; Balasankar, K.; Sathish, S.; Prabu, D.; Pushkala, D.P.; Marraiki, N.; Ramu, A.G.; et al. Effect of Ca2+ ions on naphthalene adsorption/desorption onto calcium oxide nanoparticle: Adsorption isotherm, kinetics and regeneration studies. Environ. Res. 2022, 204, 112070. [Google Scholar] [CrossRef]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.H.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Martín Lara, M.A. Caracterización y Aplicación de Biomasa Residual a la Eliminación de Metales Pesados. Ph.D. Thesis, Universidad de Granada, Facultad de Ciencias, Granada, España, 2008. [Google Scholar]
- Carrión Salazar, B.E. Definir una Técnica para la Reducción de Cromo Hexavalente en Solución Acuosa Mediante la Utilización de Musa Cavendish (Cáscara de Plátano). Ph.D. Thesis, Escuela Superior Politécnica de Chimborazo, Riobamba, Ecuador, 2016. [Google Scholar]
- Vizcaíno Mendoza, L.; Fuentes Molina, N. Biosorción de Cd, Pb y Zn por biomasa pretratada de algas rojas, cáscara de naranja y tuna. Cienc. Ing. Neogranad. 2015, 25, 43–60. [Google Scholar]
- Lasheen, M.R.; Ammar, N.S.; Ibrahim, H.S. Adsorption/desorption of Cd(II), Cu(II) and Pb(II) using chemically modified orange peel: Equilibrium and kinetic studies. Solid State Sci. 2012, 14, 202–210. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC); World Health Organization (WHO). Available online: https://monographs.iarc.fr/list-of-classifications (accessed on 27 July 2021).
- Hopkins, D.; Hawboldt, K. Biochar for the removal of metals from solution: A review of lignocellulosic and novel marine feedstocks. J. Environ. Chem. Eng. 2020, 8, 103975. [Google Scholar] [CrossRef]
- Syeda, H.I.; Sultan, I.; Razavi, K.S.; Yap, P.-S. Biosorption of heavy metals from aqueous solution by various chemically modified agricultural wastes: A review. J. Water Process. Eng. 2022, 46, 102446. [Google Scholar] [CrossRef]
- Pandey, R.; Ansari, N.G.; Prasad, R.L.; Murthy, R.C. Removal of Cd(II) ions from simulated wastewater by HCl modified Cucumis sativus peel: Equilibrium and kinetic study. Air Soil Water Res. 2014, 7, 93–101. [Google Scholar] [CrossRef]
- Bartczak, P.; Norman, M.; Klapiszewski, L.; Karwanska, N.; Kawalec, M.; Baczynska, M.; Wysokowski, M.; Zdarta, J.; Ciesielczyk, F.; Jesionowski, T. Removal of nickel(II) and lead(II) ions from aqueous solution using peat as a low-cost adsorbent: A kinetic and equilibrium study. Arab. J. Chem. 2018, 11, 1209–1222. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.S.; Ramalingam, S.; Kirupha, S.D.; Murugesan, A.; Vidhyadevi, T.; Sivanesan, S. Adsorption behavior of nickel(II) onto cashew nut shell: Equilibrium, thermodynamics, kinetics, mechanism and process design. Chem. Eng. J. 2011, 167, 122–131. [Google Scholar] [CrossRef]
- Morosanu, I.; Teodosiu, C.; Paduraru, C.; Ibanescu, D.; Tofan, L. Biosorption of lead ions from aqueous effluents by rapeseed biomass. New Biotechnol. 2017, 39, 110–124. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Adsorption of Lead on Cucumber Peel. J. Clean. Prod. 2017, 151, 603–615. [Google Scholar] [CrossRef]
- Fontana, K.B.; Lenzi, G.G.; Watanabe, E.R.L.R.; Lenzi, E.K.; Pietrobelli, J.A.M.T.; Chaves, E.S. Biosorption and Diffusion Modeling of Pb(II) by Malt Bagasse. Int. J. Chem. Eng. 2016, 2016, 4210561. [Google Scholar] [CrossRef] [Green Version]
- Janyasuthiwong, S.; Phiri, S.M.; Kijjanapanich, P.; Rene, E.R.; Esposito, G.; Lens, P.N.L. Copper, lead and zinc removal from metal-contaminated wastewater by adsorption onto agricultural wastes. Environ. Technol. 2015, 36, 3071–3083. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Biosorptive removal of lead by lentil husk. J. Environ. Chem. Eng. 2015, 3, 1088–1095. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Tao, Y.; Yu, Y.; Jiang, H.; Lian, H. Comparative study of adsorption of Pb(II) on native garlic peel and mercerized garlic peel. Environ. Sci. Pollut. Res. 2014, 21, 2054–2063. [Google Scholar] [CrossRef]
- Huang, K.; Zhu, H. Removal of Pb2+ from aqueous solution by adsorption on chemically modified muskmelon peel. Environ. Sci. Pollut. Res. 2013, 20, 4424–4434. [Google Scholar] [CrossRef]
- Cardona Gutiérrez, A.F.; Cabañas Vargas, D.D.; Zepeda Pedreguera, A. Evaluación del poder biosorbente de cáscara de naranja para la eliminación de metales pesados, Pb (II) y Zn (II). Ingeniería 2013, 17, 1–9. [Google Scholar]
- Ofomaja, A.E.; Naidoo, E.B. Biosorption of lead(II) onto pine cone powder: Studies on biosorption performance and process design to minimize biosorbent mass. Carbohydr. Polym. 2010, 82, 1031–1042. [Google Scholar] [CrossRef]
- Azmy, H.A.M.A.; Razuki, N.A.; Aziz, A.W.; Satar, N.S.A.; Kaus, N.H.M. Visible light photocatalytic activity of BiFeO3 nanoparticles for degradation of methylene blue. J. Phys. Sci. 2017, 28, 85–103. [Google Scholar] [CrossRef] [Green Version]
- Qu, R.; Sun, C.; Ma, F.; Zhang, Y.; Ji, C.; Yin, P. Removal of Fe(III) from ethanol solution by silica-gel supported dendrimer-like polyamidoamine polymers. Fuel 2018, 219, 205–213. [Google Scholar] [CrossRef]
- Herrera-Barros, A.; Tejada-Tovar, C.; Villabona-Ortíz, A.; González-Delgado, A.D.; Benítez-Monroy, J. Cd (II) and Ni (II) uptake by novel biosorbent prepared from oil palm residual biomass and Al2O3 nanoparticles. Sustain. Chem. Pharm. 2020, 15, 100216. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kumari, D.; Joshi, M.; Kyoungjin An, A.; Kumar, M. Low-cost bio-based sustainable removal of lead and cadmium using a polyphenolic bioactive Indian curry leaf (Murraya koengii) powder. Int. J. Hyg. Environ. Health 2020, 226, 113471. [Google Scholar] [CrossRef]
- Ding, Y.; Jing, D.; Gong, H.; Zhou, L.; Yang, X. Biosorption of aquatic cadmium(II) by unmodified rice straw. Bioresour. Technol. 2012, 114, 20–25. [Google Scholar] [CrossRef]
- Feng, N.; Guo, X.; Liang, S.; Zhu, Y.; Liu, J. Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J. Hazard. Mater. 2011, 185, 49–54. [Google Scholar] [CrossRef]
- Anwar, J.; Shafique, U.; Zaman, W.; Salman, M.; Dar, A.; Anwar, S. Removal of Pb(II) and Cd(II) from water by adsorption on peels of banana. Bioresour. Technol. 2010, 101, 1752–1755. [Google Scholar] [CrossRef]
- Hernández López, J. Separación de Cobalto Presente en Solución Acuosa Utilizando Cáscara de Tamarindo Como Biosorbente. Trabajo de Grado, Tecnológico de Estudios Superiores de San Felipe Del Progreso, San Felipe del Progreso, Mexico, 2014. [Google Scholar]
- Herrera-Barros, A.; Tejada-Tovar, C.; Villabona-Ortíz, A.; González-Delgado, A.D.; Fornaris-Lozada, L. Effect of pH and particle size for lead and nickel uptake from aqueous solution using cassava (Manihot esculenta) and yam (Dioscoreaalata) residual biomasses modified with titanium dioxide nanoparticles. Indian J. Sci. Technol. 2018, 11, 1–7. [Google Scholar] [CrossRef]
- Bhatnagara, A.; Minochaa, A.K. Biosorption optimization of nickel removal from water using Punica granatum peel waste. Colloids Surf. B 2010, 76, 544–548. [Google Scholar] [CrossRef]
- Bustamante Alcántara, E. Adsorción de Metales Pesados en Residuos de Café Modificados Químicamente. Ph.D. Thesis, Universidad Autónoma de Nuevo León, Facultad de Ciencias Químicas, San Nicolás de los Garza, Mexico, 2011. [Google Scholar]
- Chimanlal, I.; Lesaoana, M.; Richards, H. Chemical modification of Macadamia-derived activated carbon for remediation of selected heavy metals from wastewater. Miner. Eng. 2022, 184, 107663. [Google Scholar] [CrossRef]
- Kouassi, N.L.B.; N’goran, K.P.D.A.; Blonde, L.D.; Diabate, D.; Albert, T. Simultaneous removal of copper and lead from industrial effluents using corn cob activated carbon. Chem. Afr. 2022. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Y.; Cao, J.; Hu, H.; Dong, L.; Zha, J.; Su, Y.; Ruan, R.; Tao, S. Qualitative and relative distribution of Pb2+ adsorption mechanisms by biochars produced from a fluidized bed pyrolysis system under mild air oxidization conditions. J. Mol. Liq. 2021, 323, 114600. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Y.; Meng, Y.; Cao, J.; Hu, H.; Su, Y.; Dong, L.; Tao, S.; Ruan, R. Investigating the adsorption behavior and quantitative contribution of Pb2+ adsorption mechanisms on biochars by different feedstocks from a fluidized bed pyrolysis system. Environ. Res. 2020, 187, 109609. [Google Scholar] [CrossRef]
- Elmorsi, R.R.; El-Wakeel, S.T.; El-Dein, W.A.S.; Lofty, H.R.; Rashwan, W.E.; Nagah, M.; Shaaban, S.A.; Ahmed, S.A.S.; El-Sherif, Y.; Abou-El-Sherbini, K.S. Adsorption of Methylene Blue and Pb2+ by using acid-activated Posidonia oceanica waste. Sci. Rep. 2019, 9, 3356. [Google Scholar] [CrossRef]


| Biosorbent | Drying Temperature (°C) 1 |
|---|---|
| Palomino Fino grape seed (Vitis vinifera Palomino Fino) | 40 |
| Cabernet Sauvignon grape pomace (Vitis vinifera C. Sauvignon) | 42 |
| Loquat seed (Eryobotria japonica) | 60 |
| Calabrese broccoli stem (Brassica oleracea Italica) | 45 |
| Empty carob pod (Ceratonia siliqua) | 60 |
| Empty broad bean pod (Vicia faba) | 40 |
| Unripe bitter orange peel (Citrus aurantium) | 45 |
| Kumquat (Citrus japonica) | 40 |
| Valencia late orange pulp (Citrus sinensis Valencia late) | 120 |
| Canary Island banana pulp (Musa acuminata Colla (AAA)) | 120 (2 h) + 40 |
| Valencia late orange peel (Citrus sinensis Valencia late) | 40 |
| Biosorbent | Biosorption (%) 1 | |||
|---|---|---|---|---|
| Cd(II) | Co(II) | Ni(II) | Pb(II) | |
| Palomino Fino grape seed (Vitis vinifera Palomino Fino) | 30.1 | 18.5 | 13.4 | 89.0 |
| Cabernet Sauvignon grape pomace (Vitis vinifera C. Sauvignon) | 8.1 | 7.1 | 11.2 | 66.0 |
| Loquat seed (Eryobotria japonica) | 28.5 | 15.0 | 13.7 | 36.6 |
| Calabrese broccoli stem (Brassica oleracea Italica) | 48.9 | 28.4 | 24.8 | 78.1 |
| Empty carob pod (Ceratonia siliqua) | 61.0 | 20.8 | 22.1 | 83.0 |
| Empty broad bean pod (Vicia faba) | 61.7 | 40.7 | 39.7 | 91.5 |
| Unripe bitter orange peel (Citrus aurantium) | 37.3 | 30.5 | 25.5 | 65.5 |
| Kumquat (Citrus japonica) | 29.2 | 9.1 | 8.7 | 32.8 |
| Valencia late orange pulp (Citrus sinensis Valencia late) | 47.6 | 31.5 | 28.9 | 75.4 |
| Canary Island banana pulp (Musa acuminata Colla (AAA)) | 41.7 | 4.7 | 11.5 | 70.4 |
| Valencia late orange peel (Citrus sinensis Valencia late) | 65.2 | 47.9 | 49.0 | 88.2 |
| Metal | Biosorbent | Biosorption (%) | Reference |
|---|---|---|---|
| Cd(II) | Unmodified/modified oil palm bagasse with Al2O3 nanoparticles | 87%/91.2% | [43] |
| Curry leaf powder | 68.5% | [44] | |
| Charred orange peel | 99% | [24] | |
| Cucumber peel | 84.8% | [29] | |
| Chemically modified orange peel | 81.0% | [25] | |
| Rice straw | 80% | [45] | |
| Modified orange peel with NaOH and CaCl2 | 93.6% | [46] | |
| Banana peel | 89.2% | [47] | |
| Co(II) | Tamarind peel | 6.8% | [48] |
| Ni(II) | Unmodified/Modified oil palm bagasse with Al2O3 nanoparticles | 81%/86.9% | [43] |
| Modified sweet potato peel with TiO2 | 86.7% | [49] | |
| Modified cassava peel with TiO2 | 81.5% | [49] | |
| Peat | 92.5% | [30] | |
| Modified orange peel with NaOH and CaCl2 | 92.2% | [46] | |
| Cashew nut shell | 77.7% | [31] | |
| Pomegranate peel | 78% | [50] | |
| Pb(II) | Curry leaf powder | 84.7% | [44] |
| Peat | 100% | [30] | |
| Modified sweet potato peel with TiO2 | 99.8% | [49] | |
| Modified cassava peel with TiO2 | 99.8% | [49] | |
| Rapeseed | 94.5% | [32] | |
| Cucumber peel | 96% | [33] | |
| Malt bagasse | 97.7% | [34] | |
| Peanut shell | 85% | [35] | |
| Lentil husk | 98% | [36] | |
| Native garlic peel | 90% | [51] | |
| Mercerized garlic peel with NaOH | 97% | [37] | |
| Modified muskmelon peel with Ca(OH)2 | 100% | [38] | |
| Cross-linking orange peel with CaCl2 | 99.5% | [39] | |
| Chemically modified orange peel | 99.5% | [25] | |
| Modified coffee waste with citric acid 0.6 M | 70% | [51] | |
| Banana peel | 85.3% | [47] | |
| Pine cone power | 99.9% | [40] | |
| Unmodified/Modified Macadamia-derived activated carbon | 74.7%/87.4% | [52] | |
| Corn cob activated carbon | 77.5% | [53] | |
| Modified/Unmodified corn stalk biochar | 78.8%/83.8% | [54] | |
| Modified/Unmodified rice stalk biochar | 91.20% | [55] | |
| Acid-activated seagrass Posidonia oceanica | >95% | [56] |
| Biosorbent | Cd(II) 1 | Co(II) 1 | Ni(II) 1 | Pb(II) 1 | ||||
|---|---|---|---|---|---|---|---|---|
| Qt | Qt’ (×102) | Qt | Qt’ (×102) | Qt | Qt’ (×102) | Qt | Qt’ (×102) | |
| Grape seed | 3.39 | 3.01 | 1.16 | 1.97 | 0.79 | 1.34 | 17.38 | 8.39 |
| Grape pomace | 0.34 | 0.30 | 0.28 | 0.48 | 0.54 | 0.91 | 15.12 | 7.30 |
| Loquat seed | 1.91 | 1.70 | 0.87 | 1.47 | 0.71 | 1.21 | 6.65 | 3.21 |
| Broccoli stem | 3.19 | 2.83 | 1.48 | 2.52 | 1.31 | 2.23 | 14.17 | 6.84 |
| Empty carob pod | 6.89 | 6.13 | 1.22 | 2.07 | 1.17 | 1.99 | 15.10 | 7.29 |
| Empty broad bean pod | 8.92 | 7.93 | 2.33 | 3.95 | 2.09 | 3.56 | 15.62 | 7.54 |
| Unripe bitter Orange peel | 4.15 | 3.69 | 1.99 | 3.38 | 1.66 | 2.82 | 12.48 | 6.02 |
| Kumquat | 3.21 | 2.86 | 0.55 | 0.94 | 0.58 | 0.99 | 5.91 | 2.85 |
| Orange pulp | 2.32 | 2.07 | 1.64 | 2.78 | 1.39 | 2.37 | 16.75 | 8.09 |
| Banana pulp | 1.76 | 1.56 | 0.13 | 0.23 | 0.55 | 0.93 | 15.97 | 7.71 |
| Orange peel | 3.14 | 2.80 | 2.50 | 4.24 | 2.36 | 4.03 | 19.60 | 9.46 |
| Metal | Cd(II) | Co(II) | Ni(II) | Pb(II) |
|---|---|---|---|---|
| Cd(II) | 1.000 | |||
| Co(II) | 0.576 | 1.000 | ||
| Ni(II) | 0.557 | 0.966 | 1.000 | |
| Pb(II) | 0.124 | 0.401 | 0.477 | 1.000 |
| Biosorbent | Grape Seed | Grape Pomace | Loquat Seed | Broccoli Stem | Empty Carob Pod | Empty Broad Bean Pod | Unripe Bitter Orange Peel | Kumquat | Orange Pulp | Banana Pulp | Orange Peel |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grape seed | 1.000 | ||||||||||
| Grape pomace | 0.959 | 1.000 | |||||||||
| Loquat seed | 0.999 | 0.955 | 1.000 | ||||||||
| Broccoli stem | 0.995 | 0.982 | 0.994 | 1.000 | |||||||
| Empty carob pod | 0.834 | 0.668 | 0.826 | 0.775 | 1.000 | ||||||
| Empty broad bean pod | 0.681 | 0.466 | 0.675 | 0.603 | 0.969 | 1.000 | |||||
| Unripe bitter orange peel | 0.997 | 0.945 | 0.999 | 0.989 | 0.827 | 0.681 | 1.000 | ||||
| Kumquat | 0.722 | 0.528 | 0.712 | 0.650 | 0.984 | 0.994 | 0.714 | 1.000 | |||
| Orange pulp | 0.960 | 0.993 | 0.961 | 0.983 | 0.648 | 0.449 | 0.955 | 0.502 | 1.000 | ||
| Banana pulp | 0.985 | 0.982 | 0.978 | 0.990 | 0.794 | 0.620 | 0.968 | 0.678 | 0.966 | 1.000 | |
| Orange peel | 0.914 | 0.984 | 0.915 | 0.951 | 0.543 | 0.327 | 0.907 | 0.386 | 0.991 | 0.933 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Ponce, L.; Díaz-de-Alba, M.; Casanueva-Marenco, M.J.; Gestoso-Rojas, J.; Ortega-Iguña, M.; Galindo-Riaño, M.D.; Granado-Castro, M.D. Potential Use of Low-Cost Agri-Food Waste as Biosorbents for the Removal of Cd(II), Co(II), Ni(II) and Pb(II) from Aqueous Solutions. Separations 2022, 9, 309. https://doi.org/10.3390/separations9100309
Sánchez-Ponce L, Díaz-de-Alba M, Casanueva-Marenco MJ, Gestoso-Rojas J, Ortega-Iguña M, Galindo-Riaño MD, Granado-Castro MD. Potential Use of Low-Cost Agri-Food Waste as Biosorbents for the Removal of Cd(II), Co(II), Ni(II) and Pb(II) from Aqueous Solutions. Separations. 2022; 9(10):309. https://doi.org/10.3390/separations9100309
Chicago/Turabian StyleSánchez-Ponce, Lorena, Margarita Díaz-de-Alba, María José Casanueva-Marenco, Jesús Gestoso-Rojas, Marta Ortega-Iguña, María Dolores Galindo-Riaño, and María Dolores Granado-Castro. 2022. "Potential Use of Low-Cost Agri-Food Waste as Biosorbents for the Removal of Cd(II), Co(II), Ni(II) and Pb(II) from Aqueous Solutions" Separations 9, no. 10: 309. https://doi.org/10.3390/separations9100309
APA StyleSánchez-Ponce, L., Díaz-de-Alba, M., Casanueva-Marenco, M. J., Gestoso-Rojas, J., Ortega-Iguña, M., Galindo-Riaño, M. D., & Granado-Castro, M. D. (2022). Potential Use of Low-Cost Agri-Food Waste as Biosorbents for the Removal of Cd(II), Co(II), Ni(II) and Pb(II) from Aqueous Solutions. Separations, 9(10), 309. https://doi.org/10.3390/separations9100309