Preparation and Antioxidant Activities of Phenylethanoids from Dracocephalum heterophyllum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation and Reagents
2.2. Plant Sample Preparation and Pretreatment via Medium-Pressure Liquid Chromatography
2.3. High-Pressure Liquid Chromatography Separation and Purification of Antioxidants from Fr23 and Fr25
2.4. Purity and Antioxidant Activities of Fr2391, Fr2551, Fr2581, and Fr2582
2.5. Molecular Docking
2.6. Statistical Analysis
3. Results and Discussion
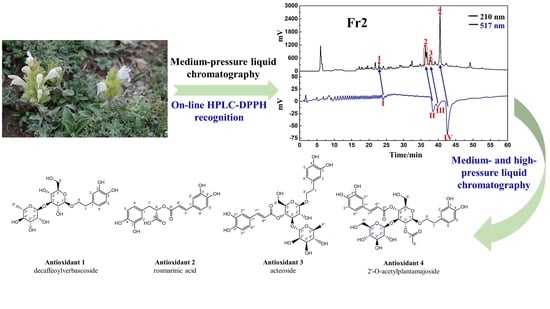
3.1. Application of Medium-Pressure Liquid Chromatography for Pretreatment of D. heterophyllum Extracts
3.2. Targeted Purification of Antioxidants Fr23 and Fr25 with High-Pressure Liquid Chromatography
3.3. Purity, Activity, and Structure of Fr2391, Fr2551, Fr2581, and Fr2582
3.4. DPPH Free Radical Scavenging Activity and Molecular Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venditti, P.; Stefano, L.D.; Meo, S.D. Mitochondrial metabolism of reactive oxygen species. Mitochondrion 2013, 13, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuca, K.; Musilek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer how are they linked? Free Radic. Biol. Med. 2010, 9, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, W.J.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The anti-Inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, D.X.; Liang, D.; Chang, C.; Jia, D.F.; Ma, F.W. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Bossis, I.; Gelasakis, A.I. Plant-Derived Natural Antioxidants in Meat and Meat Products. Antioxidants 2020, 9, 1215. [Google Scholar] [CrossRef]
- Zeng, Q.; Jin, H.Z.; Qin, J.J.; Fu, J.J.; Hu, X.J.; Liu, J.H.; Yan, L.; Chen, M.; Zhang, W.D. Chemical constituents of plants from the genus Dracocephalum. Chem. Biodivers. 2010, 7, 1911–1929. [Google Scholar] [CrossRef]
- Liu, J.Y.; He, J.X.; Shi, G.X.; An, L.Z.; Öpik, M.; Feng, H.Y. Diverse communities of arbuscular mycorrhizal fungi inhabit sites with very high altitude in Tibet Plateau. FEMS Microbiol. Ecol. 2011, 78, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Numonov, S.R.; Usmanova, S.K.; Aisa, H.A. Chemical composition of Dracocephalum heterophyllum. Chem. Nat. Compd. 2013, 49, 511–513. [Google Scholar] [CrossRef]
- Numonov, S.R.; Qureshi, M.N.; Aisa, H.A. Development of HPLC protocol and simultaneous quantification of four free flavonoids from Dracocephalum heterophyllum. Benth. Int. J. Anal. Chem. 2015, 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Wei, C.; Zhang, C.; Han, C.; Kuchkarova, N.; Shao, H. Chemical composition, phytotoxic, antimicrobial and insecticidal activity of the essential oils of Dracocephalum integrifolium. Toxins 2019, 11, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Q.Q.; Zhao, J.Q.; Dang, J.; Yuan, X.; Wang, Q.L. Triterpenes, flavonoids, and lignans from Dracocephalum heterophyllum. Chem. Nat. Compd. 2018, 54, 970–972. [Google Scholar] [CrossRef]
- Wang, L.M.; Wang, S.Q.; Yang, S.; Guo, X.J.; Lou, H.X.; Ren, D.M. Phenolic alkaloids from the aerial parts of Dracocephalum heterophyllum. Phytochemistry 2012, 82, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Li, W.; Li, H.Y.; Wang, Y.L.; Yun, T.; Song, Z.P.; Zhao, X.W. In vivo and in vitro antiviral activity of five Tibetan medicinal plant extracts against herpes simplex virus type 2 infection. Pharm Biol. 2009, 47, 598–607. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, Q.L.; Lu, X.H.; Shi, Q.Q.; Zou, J.H.; Tao, Y.D.; Wang, P. Protective effects of Dracocephalum heterophyllum in ConA-Induced acute hepatitis. Mediators Inflamm. 2016, 9, 2684321. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.Q.; Dang, J.; Wen, H.X.; Yuan, X.; Tao, Y.D.; Wang, Q.L. Anti-hepatitis, antioxidant activities and bioactive compounds of Dracocephalum heterophyllum extracts. Bot. Stud. 2016, 57, 16. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Wei, J.C.; Gao, Y.M.; Chen, R. Antioxidant and antitumoral activities of isolated macamide and macaene fractions from Lepidium meyenii (Maca). Talanta 2021, 221, 121635. [Google Scholar] [CrossRef]
- Liu, C.; Lei, Y.Q.; Dang, J.; Wang, W.D.; Zhang, J.; Mei, L.J.; Liu, Z.G.; Tao, Y.D.; Shao, Y. Preparative isolation of 1,1-diphenyl-2-picrylhydrazyl inhibitors from Ribes himalense using medium-pressure and two-dimensional reversed-phase/reversed-phase liquid chromatography guided by an online HPLC-1, 1-diphenyl-2-picrylhydrazyl assay. J. Sep. Sci. 2021, 44, 1345–1352. [Google Scholar] [CrossRef]
- Dang, J.; Wang, Q.; Wang, Q.L.; Yuan, C.; Li, G.; Ji, T.F. Preparative isolation of antioxidative gallic acid derivatives from Saxifraga tangutica using a class separation method based on medium-pressure liquid chromatography and reversed-phase liquid chromatography. J. Sep. Sci. 2021, 44, 3734–3746. [Google Scholar] [CrossRef] [PubMed]
- Li, A.F.; Xuan, H.Z.; Sun, A.L.; Liu, R.M.; Cui, J.C. Preparative separation of polyphenols from water-soluble fraction of Chinese propolis using macroporous absorptive resin coupled with preparative high performance liquid chromatography. J. Chromatogr. B 2016, 1012, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.D.; Jiao, L.J.; Tao, Y.D.; Shao, Y.; Wang, Q.L.; Yu, R.T.; Mei, L.J.; Dang, J. On-line HPLC-DPPH bioactivity-guided system for isolated of antioxidative phenylpropanoids from Qinghai-Tibet Plateau medicinal plant Lancea tibetica. J. Chromatogr. B 2019, 1106–1107, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dawa, Y.Z.; Du, Y.R.; Wang, Q.; Chen, C.B.; Zou, D.L.; Qi, D.S.; Ma, J.B.; Dang, J. Targeted isolation of 1,1-diphenyl-2-picrylhydrazyl inhibitors from Saxifraga atrata using medium- and high- pressure liquid chromatography combined with online high performance liquid chromatography-1,1-diphenyl-2-picrylhydrazyl detection. J. Chromatogr. A 2021, 1635, 461690. [Google Scholar] [CrossRef]
- Pan, G.Q.; Shen, J.W.; Ma, Y.H.; He, Y.F.; Bao, Y.; Li, R.R.; Wang, S.S.; Wang, Q.; Lin, P.C.; Dang, J. Preparative separation of isoquinoline alkaloids from Corydalis impatiens using a middle-pressure chromatogram isolated gel column coupled with two-dimensional liquid chromatography. J. Sep. Sci. 2019, 42, 3182–3190. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.P.; Wu, N.; Cao, J.Y.; Tao, Y.D.; Yu, R.T. A Method to Separate Two Main Antioxidants from Lepidium latifolium L. Extracts Using Online Medium Pressure Chromatography Tower and Two-Dimensional Inversion/Hydrophobic Interaction Chromatography Based on Online HPLC-DPPH Assay. Separations 2021, 8, 238. [Google Scholar] [CrossRef]
- İlhami, G. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Warren, J.J.; Tronic, T.A.; Mayer, J.M. Thermochemistry of Proton-Coupled Electron Transfer Reagents and its Implications. Chem. Rev. 2010, 110, 6961–7001. [Google Scholar] [CrossRef] [Green Version]
- Athar, M.; Lone, M.Y.; Khedkar, V.M.; Jha, P.C. Pharmacophore model prediction, 3D-QSAR and molecular docking studies on vinyl sulfones targeting Nrf2-mediated gene transcription intended for anti-Parkinson drug design. J. Biomol. Struct. Dyn. 2016, 34, 1282–1297. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [Green Version]
- Dang, J.; Zhang, L.; Shao, Y.; Mei, L.J.; Liu, Z.G.; Yue, H.L.; Wang, Q.L.; Tao, Y.D. Preparative isolation of antioxidative compounds from Dracocephalum heterophyllum using off-line two-dimensional reversed-phase liquid chromatography/hydrophilic interaction chromatography guided by on-line HPLC-DPPH system. J. Chromatogr. B 2018, 1095, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kanchanapoom, T.; Kasai, R.; Yamasaki, K. Phenolic glycosides from Markhamia stipulate. Phytochemistry 2002, 59, 557–563. [Google Scholar] [CrossRef]
- Dapkevicius, A.; van Beek, T.A.; Lelyveld, G.P.; van Veldhuizen, A.; de Groot, A.; Linssen, J.P.; Venskutonis, R. Isolation and structure elucidation of radical scavengers from Thymus vulgaris leaves. J. Nat. Prod. 2002, 65, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Juliao, L.D.; Piccinelli, A.L.; Marzocco, S.; Leitao, S.G.; Lotti, C.; Autore, G.; Rastrelli, L. Phenylethanoid glycosides from Lantana fucata with in vitro anti-inflammatory activity. J. Nat. Prod. 2009, 72, 1424–1428. [Google Scholar] [CrossRef]
- Kyriakopoulou, I.; Magiatis, P.; Skaltsounis, A.L.; Aligiannis, N.; Harvala, C. Samioside, a New Phenylethanoid Glycoside with Free-Radical Scavenging and Antimicrobial Activities from Phlomis samia. J. Nat. Prod. 2001, 64, 1095–1097. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Thomasa, N.S.; Georgeb, K.; Selvamc, A.A.A. Anticancer mechanism of troxerutin via targeting Nrf2 and NF-κB signaling pathways in hepatocarcinoma cell line. Toxicol. Vitro 2019, 54, 317–329. [Google Scholar] [CrossRef]
- França, A.L.d.Q.; Chaves, H.V.; Freire, J.M.d.O.; Sousa, L.H.T.d.; Pimenta, A.T.A.; Lima, M.A.S.; Oliveira, B.R.d.; Mattos, M.C.d.; Pinto, V.d.P.T.; Portela, A.M.L.R.; et al. Molecular docking study and antireabsorptive activity of a semi-synthetic coumarin derivative from Platymiscium floribundum in the ligature-induced periodontitis in rats: The involvement of heme oxygenase-1. Clin. Oral Investig. 2022, 26, 1701–1711. [Google Scholar] [CrossRef]
- Yuan, C.; Dang, J.; Han, Y.; Liu, C.; Yu, S.; Lv, Y.; Cui, Y.B.; Wang, Z.H.; Li, G. Preparative isolation of maltol glycoside from Dianthus superbus and its anti-inflammatory activity in vitro. RSC Adv. 2022, 12, 5031–5041. [Google Scholar] [CrossRef]









| Compounds | RMSD | Binding Energy (Kcal/mol) |
|---|---|---|
| Fr2391 (decaffeoylverbascoside) | 36.75 | −7.18 |
| Fr2551 (rosmarinic acid) | 37.83 | −8.29 |
| Fr2581 (acteoside) | 36.69 | −8.68 |
| Fr2582 (2′-O-acetylplantamajoside) | 35.51 | −7.85 |
| Compounds | RMSD | Binding Energy (Kcal/mol) |
|---|---|---|
| Fr2391 (decaffeoylverbascoside) | 13.49 | −4.52 |
| Fr2551 (rosmarinic acid) | 11.57 | −6.88 |
| Fr2581 (acteoside) | 12.95 | −7.17 |
| Fr2582 (2′-O-acetylplantamajoside) | 13.26 | −4.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Wang, Z.; Wu, Q.; Fang, Y.; Wang, Q.; Li, G.; Dang, J. Preparation and Antioxidant Activities of Phenylethanoids from Dracocephalum heterophyllum. Separations 2022, 9, 111. https://doi.org/10.3390/separations9050111
Lv Y, Wang Z, Wu Q, Fang Y, Wang Q, Li G, Dang J. Preparation and Antioxidant Activities of Phenylethanoids from Dracocephalum heterophyllum. Separations. 2022; 9(5):111. https://doi.org/10.3390/separations9050111
Chicago/Turabian StyleLv, Yue, Ze Wang, Qian Wu, Yan Fang, Qilan Wang, Gang Li, and Jun Dang. 2022. "Preparation and Antioxidant Activities of Phenylethanoids from Dracocephalum heterophyllum" Separations 9, no. 5: 111. https://doi.org/10.3390/separations9050111
APA StyleLv, Y., Wang, Z., Wu, Q., Fang, Y., Wang, Q., Li, G., & Dang, J. (2022). Preparation and Antioxidant Activities of Phenylethanoids from Dracocephalum heterophyllum. Separations, 9(5), 111. https://doi.org/10.3390/separations9050111