Improved Electrochemical Behavior and Thermal Stability of Li and Mn-Rich Cathode Materials Modified by Lithium Sulfate Surface Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Material Characterization
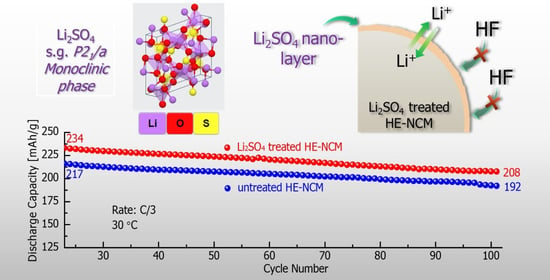
3.2. Electrochemical Behavior of HE-NCM Electrodes in Li Cells
3.3. Gas Evolution from HE-NCM Electrodes Studied by OEMS
3.4. Thermal Stability of HE-NCM Materials in Battery Solutions: DSC Studies
4. Conclusions
- A few-nanometer-thick crystalline Li2SO4 layer was formed on the material’s surface (for example, 0.35Li2MnO3.0.65LiNi0.35Mn0.45Co0.20O2) by direct and simple mixing with lithium sulfate and further annealing at 600 °C.
- From the analysis of the electrochemical data, we concluded that cathodes comprising these Li2SO4-modified materials exhibited ~20% higher discharge capacity, two-times lower charge-transfer resistance, resulting in faster kinetics, and higher capacity at various cycling rates.
- We suggest that the smooth nano-sized Li2SO4 surface layer provided mitigation of the interfacial side reactions at high anodic potentials while retaining the structural integrity of the cathode materials upon cycling in Li cells. Furthermore, we established that an additional phase, likely tetragonal spinel Li2Mn2O4, was formed during the cycling of HE-NCM cathodes due to a partial layered-to-spinel structural transformation, in agreement with the literature reports.
- A significant conclusion of this work is that the thermal stability (measured by DSC as the total heat evolution) of the Li2SO4-treated samples in reactions with EC-EMC/LiPF6 solutions decreased by ~28%. This is likely due to the artificial nano-sized Li2SO4 surface layer on the HE-NCM material, which inhibits interactions of the lattice oxygen with the solvent molecules, thus reducing heat evolution.
- We can also conclude that the Li2SO4 layer partially suppressed the electrolyte degradation during the first formation charge–discharge cycle of HE-NCM cathodes. This follows from the significantly lower CO2 and H2 gas evolution in the Li2SO4-treated samples shown by online electrochemical mass spectrometry tests.
- An important finding of this work is that the Li2SO4 phase remained on the surface of the HE-NCM cathodes and preserved its structural characteristics (space group P21/c) after 100 charge–discharge cycles.
- We propose that direct chemical treatment with nano-sized Li2SO4 for stabilizing highly reactive HE-NCM cathode materials can help to realize high-energy-density Li-ion battery systems.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thackeray, M.M.; Johnson, C.S.; Vaughey, J.T.; Li, N.; Hackney, S.A. Advances in Manganese-Oxide ‘Composite’ Electrodes for Lithium-Ion Batteries. J. Mater. Chem. 2005, 15, 2257. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Kang, S.-H.; Johnson, C.S.; Vaughey, J.T.; Benedek, R.; Hackney, S.A. Li2MnO3-Stabilized LiMO2 (M = Mn, Ni, Co) Electrodes for Lithium-Ion Batteries. J. Mater. Chem. 2007, 17, 3112. [Google Scholar] [CrossRef]
- Nayak, P.K.; Erickson, E.M.; Schipper, F.; Penki, T.R.; Munichandraiah, N.; Adelhelm, P.; Sclar, H.; Amalraj, F.; Markovsky, B.; Aurbach, D. Review on Challenges and Recent Advances in the Electrochemical Performance of High Capacity Li- and Mn-Rich Cathode Materials for Li-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702397. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen Release and Its Effect on the Cycling Stability of LiNixMnyCozO2 (NMC) Cathode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A1361–A1377. [Google Scholar] [CrossRef]
- Johnson, C.S.; Li, N.; Lefief, C.; Vaughey, J.T.; Thackeray, M.M. Synthesis, Characterization and Electrochemistry of Lithium Battery Electrodes: X Li 2 MnO 3 ·(1 − x )LiMn 0.333 Ni 0.333 Co 0.333 O 2 (0 ≤ x ≤ 0.7). Chem. Mater. 2008, 20, 6095–6106. [Google Scholar] [CrossRef]
- Zheng, J.; Myeong, S.; Cho, W.; Yan, P.; Xiao, J.; Wang, C.; Cho, J.; Zhang, J. Li- and Mn-Rich Cathode Materials: Challenges to Commercialization. Adv. Energy Mater. 2017, 7, 1601284. [Google Scholar] [CrossRef]
- Chen, G.; An, J.; Meng, Y.; Yuan, C.; Matthews, B.; Dou, F.; Shi, L.; Zhou, Y.; Song, P.; Wu, G.; et al. Cation and Anion Co-Doping Synergy to Improve Structural Stability of Li- and Mn-Rich Layered Cathode Materials for Lithium-Ion Batteries. Nano Energy 2019, 57, 157–165. [Google Scholar] [CrossRef]
- He, W.; Liu, P.; Qu, B.; Zheng, Z.; Zheng, H.; Deng, P.; Li, P. Uniform Na+ Doping-Induced Defects in Li- and Mn-Rich Cathodes for High-Performance Lithium-Ion Batteries. Adv. Sci. 2019, 6, 1802114. [Google Scholar] [CrossRef] [Green Version]
- Nayak, P.K.; Grinblat, J.; Levi, M.; Levi, E.; Kim, S.; Choi, J.W.; Aurbach, D. Al Doping for Mitigating the Capacity Fading and Voltage Decay of Layered Li and Mn-Rich Cathodes for Li-Ion Batteries. Adv. Energy Mater. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Liu, D.; Fan, X.; Li, Z.; Liu, T.; Sun, M.; Qian, C.; Ling, M.; Liu, Y.; Liang, C. A Cation/Anion Co-Doped Li1.12Na0.08Ni0.2Mn0.6O1.95F0.05 Cathode for Lithium Ion Batteries. Nano Energy 2019, 58, 786–796. [Google Scholar] [CrossRef]
- Phattharasupakun, N.; Geng, C.; Johnson, M.B.; Väli, R.; Sawangphruk, M.; Dahn, J.R. Impact of Cr Doping on the Voltage Fade of Li-Rich Mn-Rich Li1.11Ni0.33Mn0.56O2 and Li1.2Ni0.2Mn0.6O2 Positive Electrode Materials. J. Electrochem. Soc. 2020, 167, 160545. [Google Scholar] [CrossRef]
- Seok Jung, Y.; Cavanagh, A.S.; Yan, Y.; George, S.M.; Manthiram, A. Effects of Atomic Layer Deposition of Al2O3 on the Li[Li0.20Mn0.54Ni0.13Co0.13]O2 Cathode for Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, A1298. [Google Scholar] [CrossRef]
- Lei, Y.; Ni, J.; Hu, Z.; Wang, Z.; Gui, F.; Li, B.; Ming, P.; Zhang, C.; Elias, Y.; Aurbach, D.; et al. Surface Modification of Li-Rich Mn-Based Layered Oxide Cathodes: Challenges, Materials, Methods, and Characterization. Adv. Energy Mater. 2020, 10, 2002506. [Google Scholar] [CrossRef]
- Bettge, M.; Li, Y.; Sankaran, B.; Rago, N.D.; Spila, T.; Haasch, R.T.; Petrov, I.; Abraham, D.P. Improving High-Capacity Li1.2Ni 0.15 Mn0.55Co0.1O2-Based Lithium-Ion Cells by Modifiying the Positive Electrode with Alumina. J. Power Sources 2013, 233, 346–357. [Google Scholar] [CrossRef]
- Zou, G.; Yang, X.; Wang, X.; Ge, L.; Shu, H.; Bai, Y.; Wu, C.; Guo, H.; Hu, L.; Yi, X.; et al. Improvement of Electrochemical Performance for Li-Rich Spherical Li1.3[Ni0.35 Mn0.65 ]O2+x Modified by Al2O3. J. Solid State Electrochem. 2014, 18, 1789–1797. [Google Scholar] [CrossRef]
- Zhang, X.; Belharouak, I.; Li, L.; Lei, Y.; Elam, J.W.; Nie, A.; Chen, X.; Yassar, R.S.; Axelbaum, R.L. Structural and Electrochemical Study of Al2O3 and TiO2 Coated Li1.2Ni0.13Mn0.54Co0.13O = Cathode Material Using ALD. Adv. Energy Mater. 2013, 3, 1299–1307. [Google Scholar] [CrossRef]
- Gao, Y.; Patel, R.L.; Shen, K.-Y.; Wang, X.; Axelbaum, R.L.; Liang, X. Boosting the Electrochemical Performance of Li1.2Mn0.54Ni0.13Co0.13O2 by Atomic Layer-Deposited CeO2Coating. ACS Omega 2018, 3, 906–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, B.; Wang, B.; Liu, J.; Kaliyappan, K.; Sun, Q.; Liu, Y.; Dadheech, G.; Balogh, M.P.; Yang, L.; Sham, T.-K.; et al. Highly Stable Li1.2Mn0.54Co0.13Ni0.13O2Enabled by Novel Atomic Layer Deposited AlPO4 Coating. Nano Energy 2017, 34, 120–130. [Google Scholar] [CrossRef]
- Amalraj, F.; Talianker, M.; Markovsky, B.; Burlaka, L.; Leifer, N.; Goobes, G.; Erickson, E.M.; Haik, O.; Grinblat, J.; Zinigrad, E.; et al. Studies of Li and Mn-Rich Lix[MnNiCo]O2Electrodes: Electrochemical Performance, Structure, and the Effect of the Aluminum Fluoride Coating. J. Electrochem. Soc. 2013, 160, A2220–A2233. [Google Scholar] [CrossRef]
- Maiti, S.; Sclar, H.; Sharma, R.; Vishkin, N.; Fayena-Greenstein, M.; Grinblat, J.; Talianker, M.; Burstein, L.; Solomatin, N.; Tiurin, O.; et al. Understanding the Role of Alumina (Al2O3), Pentalithium Aluminate (Li5AlO4), and Pentasodium Aluminate (Na5AlO4) Coatings on the Li and Mn-Rich NCM Cathode Material 0.33Li2MnO3·0.67Li(Ni0.4Co0.2Mn0.4)O2 for Enhanced Electrochemical Performance. Adv. Funct. Mater. 2020, 31, 2008083. [Google Scholar] [CrossRef]
- Sclar, H.; Maiti, S.; Leifer, N.; Vishkin, N.; Fayena-Greenstein, M.; Hen, M.; Grinblat, J.; Talianker, M.; Solomatin, N.; Tiurin, O.; et al. Electrochemical and Thermal Behavior of Modified Li and Mn-Rich Cathode Materials in Battery Prototypes: Impact of Pentasodium Aluminate Coating and Comprehensive Understanding of Its Evolution upon Cycling through Solid-State Nuclear Magnetic Resonance A. Adv. Energy Sustain. Res. 2021, 2, 2000089. [Google Scholar] [CrossRef]
- Erickson, E.M.; Sclar, H.; Schipper, F.; Liu, J.; Tian, R.; Ghanty, C.; Burstein, L.; Leifer, N.; Grinblat, J.; Talianker, M.; et al. High-Temperature Treatment of Li-Rich Cathode Materials with Ammonia: Improved Capacity and Mean Voltage Stability during Cycling. Adv. Energy Mater. 2017, 7, 1700708. [Google Scholar] [CrossRef]
- Breddemann, U.; Erickson, E.M.; Davis, V.; Schipper, F.; Ellwanger, M.; Daub, M.; Hoffmann, A.; Erk, C.; Markovsky, B.; Aurbach, D.; et al. Fluorination of Li-Rich Lithium-Ion-Battery Cathode Materials by Fluorine Gas: Chemistry, Characterization, and Electrochemical Performance in Half Cells. ChemElectroChem 2019, 6, 3337–3349. [Google Scholar] [CrossRef]
- Ueda, M.; Ohe, M.; Kim, J.-H.; Yonezawa, S.; Takashima, M. Effects of Surface Fluorination on the Electrochemical Properties and Thermal Stability of LiFePO4 Cathode for Lithium-Ion Batteries. J. Fluor. Chem. 2013, 149, 88–94. [Google Scholar] [CrossRef]
- Qiu, B.; Zhang, M.; Wu, L.; Wang, J.; Xia, Y.; Qian, D.; Liu, H.; Hy, S.; Chen, Y.; An, K.; et al. Gas–Solid Interfacial Modification of Oxygen Activity in Layered Oxide Cathodes for Lithium-Ion Batteries. Nat. Commun. 2016, 7, 12108. [Google Scholar] [CrossRef]
- Susai, F.A.; Sclar, H.; Maiti, S.; Burstein, L.; Perkal, O.; Grinblat, J.; Talianker, M.; Ruthstein, S.; Erk, C.; Hartmann, P.; et al. Stabilized Behavior of LiNi0.85Co0.10Mn0.05O2Cathode Materials Induced by Their Treatment with SO2. ACS Appl. Energy Mater. 2020, 3, 3609–3618. [Google Scholar] [CrossRef]
- Sclar, H.; Sicklinger, J.; Erickson, E.M.; Maiti, S.; Grinblat, J.; Talianker, M.; Amalraj Susai, F.; Burstein, L.; Beyer, H.; Hartmann, L.; et al. Enhancement of Electrochemical Performance of Lithium and Manganese-Rich Cathode Materials via Thermal Treatment with SO2. J. Electrochem. Soc. 2020, 167, 110563. [Google Scholar] [CrossRef]
- Zhang, S.S.; Chen, J.; Wang, C. Elemental Sulfur as a Cathode Additive for Enhanced Rate Capability of Layered Lithium Transition Metal Oxides. J. Electrochem. Soc. 2019, 166, A487–A492. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Zeng, Y.; Gao, Z.; Xie, S.; Xiao, F.; Liu, L. Effects of Li2SO4·H2O Amounts on Morphologies of Hydrothermal Synthesized LiMnPO4Cathodes. RSC Adv. 2016, 6, 103232–103237. [Google Scholar] [CrossRef]
- Lv, D.; Wang, L.; Hu, P.; Sun, Z.; Chen, Z.; Zhang, Q.; Cheng, W.; Ren, W.; Bian, L.; Xu, J.; et al. Li2O-B2O3-Li2SO4 Modified LiNi1/3Co1/3Mn1/3O2 Cathode Material for Enhanced Electrochemical Performance. Electrochim. Acta 2017, 247, 803–811. [Google Scholar] [CrossRef]
- Nagao, K.; Hayashi, A.; Deguchi, M.; Tsukasaki, H.; Mori, S.; Tatsumisago, M. Amorphous LiCoO2Li2SO4 Active Materials: Potential Positive Electrodes for Bulk-Type All-Oxide Solid-State Lithium Batteries with High Energy Density. J. Power Sources 2017, 348, 1–8. [Google Scholar] [CrossRef]
- Savina, A.; Orlova, E.; Morozov, A.; Luchkin, S.; Abakumov, A. Sulfate-Containing Composite Based on Ni-Rich Layered Oxide LiNi0.8Mn0.1Co0.1O2 as High-Performance Cathode Material for Li-Ion Batteries. Nanomaterials 2020, 10, 2381. [Google Scholar] [CrossRef] [PubMed]
- Young, R.A.; Wiles, D.B. Profile Shape Functions in Rietveld Refinements. J. Appl. Crystallogr. 1982, 15, 430–438. [Google Scholar] [CrossRef]
- Caglioti, G.; Paoletti, A.; Ricci, F.P. Choice of Collimators for a Crystal Spectrometer for Neutron Diffraction. Nucl. Instrum. 1958, 3, 223–228. [Google Scholar] [CrossRef]
- Rosy; Sclar, H.; Evenstein, E.; Haber, S.; Maiti, S.; Sharabani, T.; Leskes, M.; Noked, M. Mitigating Structural Instability of High-Energy Lithium- and Manganese-Rich LiNixMnyCoz Oxide by Interfacial Atomic Surface Reduction. Chem. Mater. 2019, 31, 3840–3847. [Google Scholar] [CrossRef]
- GAS CHROMATOGRAPHY-DIFFERENTIAL ELECTROCHEMICAL MASS SPECTROMETRY (GC-DEMS). Available online: https://www.hidenanalytical.com/products/gas-analysis/hpr-40-dems/ (accessed on 28 February 2022).
- Peng, L.; Zhu, Y.; Khakoo, U.; Chen, D.; Yu, G. Self-Assembled LiNi1/3Co1/3Mn1/3O2 Nanosheet Cathodes with Tunable Rate Capability. Nano Energy 2015, 17, 36–42. [Google Scholar] [CrossRef]
- Croy, J.R.; Gallagher, K.G.; Balasubramanian, M.; Long, B.R.; Thackeray, M.M. Quantifying Hysteresis and Voltage Fade in XLi2MnO3(1-x)LiMn0.5Ni0.5O2Electrodes as a Function of Li2MnO3Content. J. Electrochem. Soc. 2014, 161, A318–A325. [Google Scholar] [CrossRef]
- Rama Rao, S.; Bheema Lingam, C.; Rajesh, D.; Vijayalakshmi, R.P.; Sunandana, C.S. Structural, Conductivity and Dielectric Properties of Li2SO4. Eur. Phys. J. Appl. Phys. 2014, 66, 30906. [Google Scholar] [CrossRef]
- Varghese, S.; Hariharan, K. Influence of Quenching on the Structural and Conduction Characteristics of Lithium Sulfate. Ionics 2018, 24, 2591–2599. [Google Scholar] [CrossRef]
- Assat, G.; Iadecola, A.; Foix, D.; Dedryvère, R.; Tarascan, J.-M. Direct Quantification of Anionic Redox over Long Cycling of Li-Rich NMC via Hard X-ray Photoemission Spectroscopy. ACS Energy Lett. 2018, 3, 2721–2728. [Google Scholar] [CrossRef]
- Andreu, N.; Flahaut, D.; Dedryvère, R.; Minvielle, M.; Martinez, H.; Gonbeau, D. XPS Investigation of Surface Reactivity of Electrode Materials: Effect of the Transition Metal. ACS Appl. Mater. Interfaces 2015, 7, 6629–6636. [Google Scholar] [CrossRef] [PubMed]
- Baggetto, L.; Dudney, N.J.; Veith, G.M. Surface Chemistry of Metal Oxide Coated Lithium Manganese Nickel Oxide Thin Film Cathodes Studied by XPS. Electrochim. Acta 2013, 90, 135–147. [Google Scholar] [CrossRef]
- Fantauzzi, M.; Elsener, B.; Atzei, D.; Rigoldi, A.; Rossi, A. Exploiting XPS for the Identification of Sulfides and Polysulfides. RSC Adv. 2015, 5, 75953–75963. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Rinaldo, S.G.; Gallagher, K.G.; Long, B.R.; Croy, J.R.; Bettge, M.; Dees, D.W.; Abraham, D.P.; Bare, J. Physical Theory of Voltage Fade in Lithium- and Manganese-Rich Transition Metal Oxides. 2015, 162 (6). J. Electrochem. Soc. 2015, 162, A897. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Xie, L.; He, X.; Zhuo, L.; Wang, L.; Ming, J. Electrochemical Activation, Voltage Decay and Hysteresis of Li-Rich Layered Cathode Probed by Various Cobalt Content. Electrochim. Acta 2018, 265, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; MacNeil, D.D.; Dahn, J.R. Layered Cathode Materials Li[NixLi1/3 − 2x/3Mn2/3−x/3O2 for Lithium-Ion Batteries. Electrochem. Solid-State Lett. 2001, 4, A191. [Google Scholar] [CrossRef]
- Teufl, T.; Strehle, B.; Müller, P.; Gasteiger, H.A.; Mendez, M.A. Oxygen Release and Surface Degradation of Li- and Mn-Rich Layered Oxides in Variation of the Li2MnO3Content. J. Electrochem. Soc. 2018, 165, A2718–A2731. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.; Persson, K.A. Structural and Chemical Evolution of the Layered Li-Excess LixMnO3 as a Function of Li Content from First-Principles Calculations. Adv. Energy Mater. 2014, 4, 1400498. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, D.; Kalnaus, S.; Meisner, R.A.; Rhodes, K.J.; Li, J.; Payzant, E.A.; Wood, D.L.; Daniel, C. Structural Transformation of a Lithium-Rich Li1.2Co0.1Mn0.55Ni0.15O2 Cathode during High Voltage Cycling Resolved by in Situ X-ray Diffraction. J. Power Sources 2013, 229, 239–248. [Google Scholar] [CrossRef]
- Amalraj, S.F.; Sharon, D.; Talianker, M.; Julien, C.M.; Burlaka, L.; Lavi, R.; Zhecheva, E.; Markovsky, B.; Zinigrad, E.; Kovacheva, D.; et al. Study of the Nanosized Li2MnO3: Electrochemical Behavior, Structure, Magnetic Properties, and Vibrational Modes. Electrochim. Acta 2013, 97, 259–270. [Google Scholar] [CrossRef]
- Susai, F.A.; Talianker, M.; Liu, J.; Rosy; Paul, T.; Grinblat, Y.; Erickson, E.; Noked, M.; Burstein, L.; Frenkel, A.I.; et al. Electrochemical Activation of Li2MnO3 Electrodes at 0 °C and Its Impact on the Subsequent Performance at Higher Temperatures. Materials 2020, 13, 4388. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Shi, W.; Gu, M.; Xiao, J.; Zuo, P.; Wang, C.; Zhang, J.-G. Electrochemical Kinetics and Performance of Layered Composite Cathode Material Li[Li0.2Ni0.2Mn0.6]O2. J. Electrochem. Soc. 2013, 160, A2212–A2219. [Google Scholar] [CrossRef]
- Lu, X.; Li, X.; Wang, Z.; Guo, H.; Yan, G.; Yin, X. A Modified Co-Precipitation Process to Coat LiNi1/3Co1/3Mn1/3O2 onto LiNi0.8Co0.1Mn0.1O2 for Improving the Electrochemical Performance. Appl. Surf. Sci. 2014, 297, 182–187. [Google Scholar] [CrossRef]
- Tarneberg, R. Ion Diffusion in the High-Temperature Phases Li2SO4, LiNaSO4, LiAgSO4 and Li4Zn(SO4)3. Solid State Ionics 1996, 90, 209–220. [Google Scholar] [CrossRef]
- Nord, A.G. Crystal Structure of β-Li2SO4. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1976, 32, 982–983. [Google Scholar] [CrossRef] [Green Version]
- Kleiner, K.; Strehle, B.; Baker, A.R.; Day, S.J.; Tang, C.C.; Buchberger, I.; Chesneau, F.-F.; Gasteiger, H.A.; Piana, M. Origin of High Capacity and Poor Cycling Stability of Li-Rich Layered Oxides: A Long-Duration in Situ Synchrotron Powder Diffraction Study. Chem. Mater. 2018, 30, 3656–3667. [Google Scholar] [CrossRef]
- Strehle, B.; Kleiner, K.; Jung, R.; Chesneau, F.; Mendez, M.; Gasteiger, H.A.; Piana, M. The Role of Oxygen Release from Li- and Mn-Rich Layered Oxides during the First Cycles Investigated by On-Line Electrochemical Mass Spectrometry. J. Electrochem. Soc. 2017, 164, A400–A406. [Google Scholar] [CrossRef]
- Muhammad, S.; Kim, H.; Kim, Y.; Kim, D.; Song, J.H.; Yoon, J.; Park, J.; Ahn, S.; Kang, S.; Thackeray, M.M.; et al. Evidence of Reversible Oxygen Participation in Anomalously High Capacity Li- and Mn-Rich Cathodes for Li-Ion Batteries. Nano Energy 2016, 21, 172–184. [Google Scholar] [CrossRef] [Green Version]
- Rowden, B.; Garcia-Araez, N. A Review of Gas Evolution in Lithium Ion Batteries. Energy Rep. 2020, 6, 10–18. [Google Scholar] [CrossRef]
- Sicklinger, J.; Beyer, H.; Hartmann, L.; Riewald, F.; Sedlmeier, C.; Gasteiger, H.A. SO3Treatment of Lithium- and Manganese-Rich NCMs for Li-Ion Batteries: Enhanced Robustness towards Humid Ambient Air and Improved Full-Cell Performance. J. Electrochem. Soc. 2020, 167, 130507. [Google Scholar] [CrossRef]
- Metzger, M.; Strehle, B.; Solchenbach, S.; Gasteiger, H.A. Origin of H2Evolution in LIBs: H2O Reduction vs. Electrolyte Oxidation. J. Electrochem. Soc. 2016, 163, A798–A809. [Google Scholar] [CrossRef]
- Inoue, T.; Mukai, K. Are All-Solid-State Lithium-Ion Batteries Really Safe?–Verification by Differential Scanning Calorimetry with an All-Inclusive Microcell. ACS Appl. Mater. Interfaces 2017, 9, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Haik, O.; Amalraj, F.S.; Hirshberg, D.; Burlaka, L.; Talianker, M.; Markovsky, B.; Zinigrad, E.; Aurbach, D.; Lampert, J.K.; Shin, J.-Y.; et al. Thermal Processes in the Systems with Li-Battery Cathode Materials and LiPF6 -Based Organic Solutions. J. Solid State Electrochem. 2014, 18, 2333–2342. [Google Scholar] [CrossRef]












| Material | [Li2MnO3] Monoclinic Phase | Rhombohedral Phase [Li(TM)O2] (In Terms of the Hexagonal Cell) | Li2SO4 (P21/a) Monoclinic Phase |
|---|---|---|---|
| Untreated HE-NCM | a = 4.9775 Å b = 8.5388 Å c = 5.0139 Å β = 108.90° | a = 2.8556 Å c = 14.2496 Å | |
| Li2SO4-treated HE-NCM | a = 5.0119 Å b = 8.5536 Å c = 4.9910 Å β = 108.75° | a = 2.8556 Å c = 14.2514 Å | a = 8.2225 Å b = 4.9860 Å c = 8.4187 Å β = 107.85° |
| Cycle Number | Electrochemical Cell Configuration | |||||||
|---|---|---|---|---|---|---|---|---|
| Untreated HE-NCM vs. Li/Li+ | Li2SO4-Treated HE-NCM vs. Li/Li+ | |||||||
| Rhf (Ω) | Rcon. (Ω) | Rsl (Ω) | Rct (Ω) | Rhf (Ω) | Rcon. (Ω) | Rsl (Ω) | Rct (Ω) | |
| 15th Cycle | 1.7 | 8.1 | - | 22.6 | 3.0 | 10 | - | 14.4 |
| 100th Cycle | 1.7 | 13.2 | 19.7 | 137.7 | 3.0 | 14.6 | 11 | 55.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sclar, H.; Maiti, S.; Sharma, R.; Erickson, E.M.; Grinblat, J.; Raman, R.; Talianker, M.; Noked, M.; Kondrakov, A.; Markovsky, B.; et al. Improved Electrochemical Behavior and Thermal Stability of Li and Mn-Rich Cathode Materials Modified by Lithium Sulfate Surface Treatment. Inorganics 2022, 10, 39. https://doi.org/10.3390/inorganics10030039
Sclar H, Maiti S, Sharma R, Erickson EM, Grinblat J, Raman R, Talianker M, Noked M, Kondrakov A, Markovsky B, et al. Improved Electrochemical Behavior and Thermal Stability of Li and Mn-Rich Cathode Materials Modified by Lithium Sulfate Surface Treatment. Inorganics. 2022; 10(3):39. https://doi.org/10.3390/inorganics10030039
Chicago/Turabian StyleSclar, Hadar, Sandipan Maiti, Rosy Sharma, Evan M. Erickson, Judith Grinblat, Ravikumar Raman, Michael Talianker, Malachi Noked, Aleksandr Kondrakov, Boris Markovsky, and et al. 2022. "Improved Electrochemical Behavior and Thermal Stability of Li and Mn-Rich Cathode Materials Modified by Lithium Sulfate Surface Treatment" Inorganics 10, no. 3: 39. https://doi.org/10.3390/inorganics10030039
APA StyleSclar, H., Maiti, S., Sharma, R., Erickson, E. M., Grinblat, J., Raman, R., Talianker, M., Noked, M., Kondrakov, A., Markovsky, B., & Aurbach, D. (2022). Improved Electrochemical Behavior and Thermal Stability of Li and Mn-Rich Cathode Materials Modified by Lithium Sulfate Surface Treatment. Inorganics, 10(3), 39. https://doi.org/10.3390/inorganics10030039