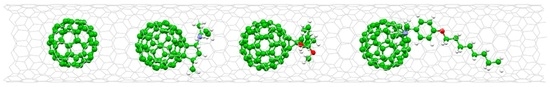
Diameter-Selective Host-Guest Interactions between Functionalized Fullerenes and Single-Walled Carbon Nanotubes
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Purification of SWCNTs
3.2. Synthesis of 1, 2 and 3
3.3. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smith, B.W.; Monthioux, M.; Luzzi, D.E. Encapsulated C60 in Carbon Nanotubes. Nature 1998, 396, 323–324. [Google Scholar] [CrossRef]
- Fakrach, B.; Fergani, F.; Boutahir, M.; Rahmani, A.; Chadli, H.; Hermet, P.; Rahmani, A. Structure and Raman Spectra of C60 and C70 Fullerenes Encased into Single-Walled Boron Nitride Nanotubes: A Theoretical Study. Crystals 2018, 8, 118. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Chai, J.; Shi, J. Nano-Peapods from C60-Encapsulated CNTs Driving Self-Assembly of Phosphorus Nanotube: A Molecular Dynamics Study. Comput. Mater. Sci. 2019, 160, 403–410. [Google Scholar] [CrossRef]
- Nishimura, M.; Hatta, M. Local Buckling Behavior of Multi-Walled Carbon Nanotubes Encapsulating C60 Fullerenes. Carbon Trends 2023, 11, 100269. [Google Scholar] [CrossRef]
- Kodama, T.; Ohnishi, M.; Park, W.; Shiga, T.; Park, J.; Shimada, T.; Shinohara, H.; Shiomi, J.; Goodson, K.E. Modulation of Thermal and Thermoelectric Transport in Individual Carbon Nanotubes by Fullerene Encapsulation. Nat. Mater 2017, 16, 892–897. [Google Scholar] [CrossRef]
- Nakanishi, R.; Satoh, J.; Katoh, K.; Zhang, H.; Breedlove, B.K.; Nishijima, M.; Nakanishi, Y.; Omachi, H.; Shinohara, H.; Yamashita, M. DySc2N@C80 Single-Molecule Magnetic Metallofullerene Encapsulated in a Single-Walled Carbon Nanotube. J. Am. Chem. Soc. 2018, 140, 10955–10959. [Google Scholar] [CrossRef]
- Okazaki, T.; Suenaga, K.; Hirahara, K.; Bandow, S.; Iijima, S.; Shinohara, H. Electronic and Geometric Structures of Metallofullerene Peapods. Phys. B Condens. Matter 2002, 323, 97–99. [Google Scholar] [CrossRef]
- Sato, Y.; Suenaga, K.; Okubo, S.; Okazaki, T.; Iijima, S. Structures of D5d-C80 and Ih-Er3N@C80 Fullerenes and Their Rotation Inside Carbon Nanotubes Demonstrated by Aberration-Corrected Electron Microscopy. Nano Lett. 2007, 7, 3704–3708. [Google Scholar] [CrossRef]
- Sloan, J.; Dunin-Borkowski, R.E.; Hutchison, J.L.; Coleman, K.S.; Clifford Williams, V.; Claridge, J.B.; York, A.P.E.; Xu, C.; Bailey, S.R.; Brown, G.; et al. The Size Distribution, Imaging and Obstructing Properties of C60 and Higher Fullerenes Formed within Arc-Grown Single Walled Carbon Nanotubes. Chem. Phys. Lett. 2000, 316, 191–198. [Google Scholar] [CrossRef]
- Wilson, M.; Madden, P.A. Growth of Ionic Crystals in Carbon Nanotubes. J. Am. Chem. Soc. 2001, 123, 2101–2102. [Google Scholar] [CrossRef]
- Kavan, L.; Dunsch, L.; Kataura, H.; Oshiyama, A.; Otani, M.; Okada, S. Electrochemical Tuning of Electronic Structure of C60 and C70 Fullerene Peapods: In Situ Visible Near-Infrared and Raman Study. J. Phys. Chem. B 2003, 107, 7666–7675. [Google Scholar] [CrossRef]
- Yang, X.; Yao, M.; Wu, X.; Liu, S.; Chen, S.; Yang, K.; Liu, R.; Cui, T.; Sundqvist, B.; Liu, B. Novel Superhard Sp3 Carbon Allotrope from Cold-Compressed C70 Peapods. Phys. Rev. Lett. 2017, 118, 245701. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Okubo, S.; Nakanishi, T.; Joung, S.-K.; Saito, T.; Otani, M.; Okada, S.; Bandow, S.; Iijima, S. Optical Band Gap Modification of Single-Walled Carbon Nanotubes by Encapsulated Fullerenes. J. Am. Chem. Soc. 2008, 130, 4122–4128. [Google Scholar] [CrossRef]
- Yang, J.; Lee, J.; Lee, J.; Yi, W. Conductivity and Field Emission Enhancement of C60-Encapsulated Single-Walled Carbon Nanotubes. Diam. Relat. Mater. 2018, 89, 273–281. [Google Scholar] [CrossRef]
- Matsuno, T.; Terasaki, S.; Kogashi, K.; Katsuno, R.; Isobe, H. A Hybrid Molecular Peapod of Sp2- and Sp3-Nanocarbons Enabling Ultrafast Terahertz Rotations. Nat. Commun. 2021, 12, 5062. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Kahng, S.-J.; Kim, G.; Son, Y.-W.; Ihm, J.; Kato, H.; Wang, Z.W.; Okazaki, T.; Shinohara, H.; et al. Bandgap Modulation of Carbon Nanotubes by Encapsulated Metallofullerenes. Nature 2002, 415, 1005–1008. [Google Scholar] [CrossRef]
- Kuznetsov, V. Stereochemistry of Simple Molecules inside Nanotubes and Fullerenes: Unusual Behavior of Usual Systems. Molecules 2020, 25, 2437. [Google Scholar] [CrossRef]
- Hornbaker, D.J.; Kahng, S.-J.; Misra, S.; Smith, B.W.; Johnson, A.T.; Mele, E.J.; Luzzi, D.E.; Yazdani, A. Mapping the One-Dimensional Electronic States of Nanotube Peapod Structures. Science 2002, 295, 828–831. [Google Scholar] [CrossRef]
- Rodríguez Méndez, Á.; Medrano Sandonas, L.; Dianat, A.; Gutierrez, R.; Cuniberti, G. An Atomistic Study of the Thermoelectric Signatures of CNT Peapods. J. Phys. Chem. C 2021, 125, 13721–13731. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, J.-W. Modulation of Thermal Conductivity of Single-Walled Carbon Nanotubes by Fullerene Encapsulation: The Effect of Vacancy Defects. Phys. Chem. Chem. Phys. 2023, 25, 7734–7740. [Google Scholar] [CrossRef]
- Kaur, R.; Sen, S.; Larsen, M.C.; Tavares, L.; Kjelstrup-Hansen, J.; Ishida, M.; Zieleniewska, A.; Lynch, V.M.; Bähring, S.; Guldi, D.M.; et al. Semiconducting Supramolecular Organic Frameworks Assembled from a Near-Infrared Fluorescent Macrocyclic Probe and Fullerenes. J. Am. Chem. Soc. 2020, 142, 11497–11505. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chamberlain, T.W.; Wang, Y.; Yang, S.; Blake, A.J.; Schröder, M.; Khlobystov, A.N. Encapsulation of Transition Metal Atoms into Carbon Nanotubes: A Supramolecular Approach. Chem. Commun. 2011, 47, 5696–5698. [Google Scholar] [CrossRef] [PubMed]
- Avdoshenko, S.M.; Fritz, F.; Schlesier, C.; Kostanyan, A.; Dreiser, J.; Luysberg, M.; Popov, A.A.; Meyer, C.; Westerström, R. Partial Magnetic Ordering in One-Dimensional Arrays of Endofullerene Single-Molecule Magnet Peapods. Nanoscale 2018, 10, 18153–18160. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, T.W.; Pfeiffer, R.; Howells, J.; Peterlik, H.; Kuzmany, H.; Kräutler, B.; Ros, T.D.; Melle-Franco, M.; Zerbetto, F.; Milić, D.; et al. Engineering Molecular Chains in Carbon Nanotubes. Nanoscale 2012, 4, 7540–7548. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Yamasaki, T.; Isobe, H. Solid-State Structures of Peapod Bearings Composed of Finite Single-Wall Carbon Nanotube and Fullerene Molecules. Proc. Natl. Acad. Sci. USA 2014, 111, 8374–8379. [Google Scholar] [CrossRef]
- Fergani, F.; Chadli, H.; Belhboub, A.; Hermet, P.; Rahmani, A. Theoretical Study of the Raman Spectra of C70 Fullerene Carbon Peapods. J. Phys. Chem. C 2015, 119, 5679–5686. [Google Scholar] [CrossRef]
- Bandow, S.; Hiraoka, T.; Yumura, T.; Hirahara, K.; Shinohara, H.; Iijima, S. Raman Scattering Study on Fullerene Derived Intermediates Formed within Single-Wall Carbon Nanotube: From Peapod to Double-Wall Carbon Nanotube. Chem. Phys. Lett. 2004, 384, 320–325. [Google Scholar] [CrossRef]
- Joung, S.-K.; Okazaki, T.; Kishi, N.; Okada, S.; Bandow, S.; Iijima, S. Effect of Fullerene Encapsulation on Radial Vibrational Breathing-Mode Frequencies of Single-Wall Carbon Nanotubes. Phys. Rev. Lett. 2009, 103, 027403. [Google Scholar] [CrossRef]
- Okubo, S.; Okazaki, T.; Kishi, N.; Joung, S.-K.; Nakanishi, T.; Okada, S.; Iijima, S. Diameter-Dependent Band Gap Modification of Single-Walled Carbon Nanotubes by Encapsulated Fullerenes. J. Phys. Chem. C 2009, 113, 571–575. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman Spectroscopy of Carbon Nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Chen, M.; Shen, W.; Bao, L.; Cai, W.; Xie, Y.; Akasaka, T.; Lu, X. Regioselective Thermal Reaction between Triethylamine and C60 Revisited: X-Ray Confirmation of the Pentane-Fused Adduct and in Situ Mechanism Study. Eur. J. Org. Chem. 2015, 2015, 5742–5746. [Google Scholar] [CrossRef]
- Simon, F.; Kuzmany, H.; Rauf, H.; Pichler, T.; Bernardi, J.; Peterlik, H.; Korecz, L.; Fülöp, F.; Jánossy, A. Low Temperature Fullerene Encapsulation in Single Wall Carbon Nanotubes: Synthesis of N@C60@SWCNT. Chem. Phys. Lett. 2004, 383, 362–367. [Google Scholar] [CrossRef]
- Akasaka, T.; Wakahara, T.; Maeda, Y.; Kataura, H.; Maruyama, S. Processing Method of Single-Walled Carbon Nanotube. J.P. Patent No. 4792591B2, 12 October 2011. [Google Scholar]
- Rojwal, V.; Singha, M.K.; Mondal, T.K. Amorphous Silicon and Carbon Nanotubes Layered Thin-Film Based Device for Temperature Sensing Application. IEEE Sens. J. 2021, 21, 2627–2633. [Google Scholar] [CrossRef]
- Zeinabad, H.A.; Zarrabian, A.; Saboury, A.A.; Alizadeh, A.M.; Falahati, M. Interaction of Single and Multi Wall Carbon Nanotubes with the Biological Systems: Tau Protein and PC12 Cells as Targets. Sci. Rep. 2016, 6, 26508. [Google Scholar] [CrossRef] [PubMed]





| Samples | The RBM Peaks (cm−1) and Calculated Diameters (nm) of the SWCNTs | ||||
|---|---|---|---|---|---|
| SWCNT | 131.22 (1.89) | 150.30 (1.65) | 183.70 (1.35) | ||
| C60@SWCNT | 131.22 (1.89) | 152.15 (1.63) | 172.22 (1.44) | 183.70 (1.35) | |
| 1@SWCNT | 131.22 (1.89) | 151.22 (1.64) | 178.42 (1.39) | 195.28 (1.27) | |
| 2@SWCNT | 131.22 (1.89) | 162.22 (1.64) | 174.64 (1.42) | 190.77 (1.30) | |
| 3@SWCNT | 131.22 (1.89) | 149.40 (1.66) | 181.02 (1.37) | 195.28 (1.27) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Gao, W.; Sun, C.; Liu, Y.; Lu, X.; Lu, X. Diameter-Selective Host-Guest Interactions between Functionalized Fullerenes and Single-Walled Carbon Nanotubes. Inorganics 2023, 11, 386. https://doi.org/10.3390/inorganics11100386
Zhang R, Gao W, Sun C, Liu Y, Lu X, Lu X. Diameter-Selective Host-Guest Interactions between Functionalized Fullerenes and Single-Walled Carbon Nanotubes. Inorganics. 2023; 11(10):386. https://doi.org/10.3390/inorganics11100386
Chicago/Turabian StyleZhang, Rui, Wanru Gao, Chang Sun, Yiwen Liu, Xiaojun Lu, and Xing Lu. 2023. "Diameter-Selective Host-Guest Interactions between Functionalized Fullerenes and Single-Walled Carbon Nanotubes" Inorganics 11, no. 10: 386. https://doi.org/10.3390/inorganics11100386
APA StyleZhang, R., Gao, W., Sun, C., Liu, Y., Lu, X., & Lu, X. (2023). Diameter-Selective Host-Guest Interactions between Functionalized Fullerenes and Single-Walled Carbon Nanotubes. Inorganics, 11(10), 386. https://doi.org/10.3390/inorganics11100386