Preparing a Ca-Bi-O System by the Precipitation Method and Studying Its Intermediate Structural Properties for Applications in Water Treatment
Abstract
:1. Introduction
2. Results
2.1. X-ray Diffraction
2.2. FT-IR Analysis
- All samples present broad absorption bands at 3426 cm−1 and 1635 cm−1 due to the stretching and scissoring vibrations of the adsorbed water;
- The peaks from 1422 cm−1, 873 cm−1, and 713 cm−1 are specific for asymmetric C-O stretching, out-of-plane vibration, and in-plane vibration from the traces of trapped carbonate-based compounds that result from the decomposition of oxalates, respectively. This decreases with temperature [31]. The peak from 1795 cm−1 can also be attributed to calcite [32];
- The 400–650 cm−1 domain, attributed to the Bi-O and Ca-O vibrations, shows that between 450 and 650 cm−1 a structuring of the crystal lattice takes place. The IR absorption spectra of the samples contained additional absorption bands specifically for Ca2Bi2O5 at 428, 449, 492, 522, and 622 cm−1, corresponding to the vibrations of the Bi–O and Ca–O bonds [33];
- The peak from 3640 cm−1 can be attributed to the OH stretching vibration from a trace of the hydroxy compound formed with water from the atmosphere [31,34]. When CBO particles’ highly reactive surface areas are exposed to air during calcination, CO2 and H2O are formed and then adsorbed as free -OH and carbonate species on the CBO surface. The stretching vibration of water can also indicate the adsorptive properties of the materials. In the heterogeneous catalysis, adsorption is an important step as it precedes the chemical reaction on the catalyst surface and facilitates the reaction path on the active sites [35,36].
2.3. Raman Shift Spectroscopy
2.4. Ultraviolet-Visible Spectroscopy and Ab Initio Calculations
2.5. Scanning Electron Microscopy Coupled with Energy Dispersive Spectral Examination and Inductively Coupled Plasma—Optical Emission Spectrometry Analysis
2.6. Thermal Analysis
2.7. Catalytic Experiments
3. Discussion
4. Materials and Methods
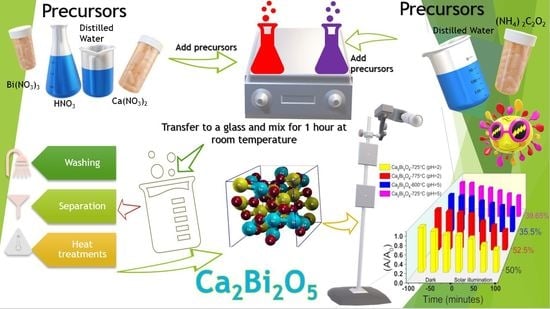
4.1. Preparation
4.2. Characterization Techniques
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okello, C.; Tomasello, B.; Greggio, N.; Wambiji, N.; Antonellini, M. Impact of Population Growth and Climate Change on the Freshwater Resources of Lamu Island, Kenya. Water 2015, 7, 1264–1290. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, S.; Li, X.; Wu, X.; Yang, C.; Zeng, G.; Peng, Y.; Zhou, Q.; Lu, L. Microstructure and Performance of Z-Scheme Photocatalyst of Silver Phosphate Modified by MWCNTs and Cr-Doped SrTiO3 for Malachite Green Degradation. Appl. Catal. B Environ. 2018, 227, 557–570. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, X.; Han, Y.; Yang, C.; Ma, Y.; Du, C.; Teng, Q.; Liu, H.; Zhong, Y. Spatial Separation of Photogenerated Carriers and Enhanced Photocatalytic Performance on Ag3PO4 Catalysts via Coupling with PPy and MWCNTs. Appl. Catal. B Environ. 2019, 258, 117969. [Google Scholar] [CrossRef]
- Martínez, J.; Cortés, J.F.; Miranda, R. Green Chemistry Metrics, A Review. Processes 2022, 10, 1274. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Environmental Impacts of Food Production. Available online: https://ourworldindata.org/environmental-impacts-of-food (accessed on 9 December 2022).
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Safitri, Y.A.; Indrawan, I.W.A.; Winarsih, S. Rhodamine B Induces Oxidative Stress and Cervical Epithelial Cell Proliferation in the Uterus. Toxicol. Rep. 2015, 2, 1434–1436. [Google Scholar] [CrossRef]
- Maryantï, S.A.; Sucïatï, S.; Wahyunï, E.S.; Santoso, S.; Wïyasa, İ.W.A. Rhodamine B Triggers Ovarian Toxicity Through Oxidative Stress. Cukurova Med. J. 2014, 39, 451–457. [Google Scholar] [CrossRef]
- Atri, S.; Malik, V.; Uma, S.; Nagarajan, R. Catalytic Applications of Mesoporous CaBi2O4 Obtained from a Single Source Precursor. Res. Chem. Intermed. 2019, 45, 2457–2470. [Google Scholar] [CrossRef]
- Tang, J.; Zou, Z.; Ye, J. Efficient Photocatalytic Decomposition of Organic Contaminants over CaBi2O4 under Visible-Light Irradiation. Angew. Chem. Int. Ed. 2004, 43, 4463–4466. [Google Scholar] [CrossRef]
- Rokesh, K.; Sakar, M.; Do, T.O. Calcium Bismuthate (CaBiO3): A Potential Sunlight-Driven Perovskite Photocatalyst for the Degradation of Emerging Pharmaceutical Contaminants. ChemPhotoChem 2020, 4, 373–380. [Google Scholar] [CrossRef]
- Khosya, M.; Faraz, M.; Khare, N. Enhanced Photocatalytic Reduction of Hexavalent Chromium by Using Piezo-Photo Active Calcium Bismuth Oxide Ferroelectric Nanoflakes. New J. Chem. 2022, 46, 12244–12251. [Google Scholar] [CrossRef]
- Parise, J.B.; Torardi, C.C.; Rawn, C.J.; Roth, R.S.; Burton, B.P.; Santoro, A. Synthesis and Structure of Ca6Bi6O15: Its Relationship to Ca4Bi6O13. J. Solid State Chem. 1993, 102, 132–139. [Google Scholar] [CrossRef]
- Luo, W.; Tang, J.; Zou, Z.; Ye, J. Preparation and Photophysical Properties of Some Oxides in Ca–Bi–O System. J. Alloys Compd. 2008, 455, 346–352. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Li, T.; Cai, J.; Luo, M.; Zhao, L. Novel CaBi6O10 Photocatalyst for Methylene Blue Degradation under Visible Light Irradiation. Catal. Commun. 2012, 18, 161–164. [Google Scholar] [CrossRef]
- Li, W.; Kong, D.; Cui, X.; Du, D.; Yan, T.; You, J. Hydrothermal Synthesis of Ca3Bi8O15 Rods and Their Visible Light Photocatalytic Properties. Mater. Res. Bull. 2014, 51, 69–73. [Google Scholar] [CrossRef]
- Kumada, N.; Xu, N.; Miura, A.; Takei, T. Preparation and Photocatalytic Properties of New Calcium and Lead Bismuthates. J. Ceram. Soc. Jpn. 2014, 122, 509–512. [Google Scholar] [CrossRef]
- Fang, L.; Jiang, X.W.; Guo, H.H. Ultralow Sintering Temperature Stable Type Microwave Dielectric Ceramic Ca5Bi14O26 and Preparation Method Thereof. CN103496972A, 8 January 2014. [Google Scholar]
- Solarska, R.; Heel, A.; Ropka, J.; Braun, A.; Holzer, L.; Ye, J.; Graule, T. Nanoscale Calcium Bismuth Mixed Oxide with Enhanced Photocatalytic Performance under Visible Light. Appl. Catal. Gen. 2010, 382, 190–196. [Google Scholar] [CrossRef]
- Ji, X.; Lu, J.-F.; Wang, Q.; Zhang, D. Impurity Doping Approach on Bandgap Narrowing and Improved Photocatalysis of Ca2Bi2O5. Powder Technol. 2020, 376, 708–723. [Google Scholar] [CrossRef]
- Ji, X.; Wang, Q.; Lu, J.-F.; Zhang, D. Construction of a Novel Ca2Bi2O5/α-Bi2O3 Semiconductor Heterojunction for Enhanced Visible Photocatalytic Application. Ceram. Int. 2020, 46, 13630–13640. [Google Scholar] [CrossRef]
- Shtarev, D.S.; Ryabchuk, V.K.; Rudakova, A.V.; Shtareva, A.V.; Molokeev, M.S.; Kirichenko, E.A.; Serpone, N. Phenomenological Rule from Correlations of Conduction/Valence Band Energies and Bandgap Energies in Semiconductor Photocatalysts: Calcium Bismuthates versus Strontium Bismuthates. ChemCatChem 2020, 12, 1551–1555. [Google Scholar] [CrossRef]
- Zyryanov, V.V. Mechanochemical Synthesis and Thermal Behavior of Metastable Mixed Oxides in the CaO–Sb2O3–Bi2O3 System. Inorg. Mater. 2003, 39, 1163–1171. [Google Scholar] [CrossRef]
- Rawn, C.J.; Roth, R.S.; McMurdie, H.F. Powder X-Ray Diffraction Data for Ca2Bi2O5and C4Bi6O13. Powder Diffr. 1992, 7, 109–111. [Google Scholar] [CrossRef]
- Roth, R.S.; Hwang, N.M.; Rawn, C.J.; Burton, B.P.; Ritter, J.J. Phase Equilibria in the Systems CaO–CuO and CaO-Bi2O3. J. Am. Ceram. Soc. 1991, 74, 2148–2151. [Google Scholar] [CrossRef]
- Burton, B.P.; Rawn, C.J.; Roth, R.S.; Hwang, N.M. Phase Equilibria and Crystal Chemistry in Portions of the System SrO-CaO-Bi2O3-CuO, Part IV- The System CaO-Bi2O3-CuO. J. Res. Natl. Inst. Stand. Technol. 1993, 98, 469–516. [Google Scholar] [CrossRef]
- Wang, H.Y.; Xiong, K.; Hu, Y.C.; Zhang, K.L.; Xiong, R. The Morphology Effect on Ca-Bi-O System Photocatalytic Property under Visible-Light Irradiation. Key Eng. Mater. 2013, 538, 219–222. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid Functionals Based on a Screened Coulomb Potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Thongkam, M.; Saelim, J.; Boonchom, B.; Seesanong, S.; Chaiseeda, K.; Laohavisuti, N.; Bunya-atichart, K.; Boonmee, W.; Taemchuay, D. Simple and Rapid Synthesis of Calcium Acetate from Scallop Shells to Reduce Environmental Issues. Adsorpt. Sci. Technol. 2021, 2021, 6450289. [Google Scholar] [CrossRef]
- dos Santos, V.H.J.M.; Pontin, D.; Ponzi, G.G.D.; Stepanha, A.S.D.G.; Martel, R.B.; Schütz, M.K.; Einloft, S.M.O.; Dalla Vecchia, F. Application of Fourier Transform Infrared Spectroscopy (FTIR) Coupled with Multivariate Regression for Calcium Carbonate (CaCO3) Quantification in Cement. Constr. Build. Mater. 2021, 313, 125413. [Google Scholar] [CrossRef]
- Kiefer, J.; Stärk, A.; Kiefer, A.; Glade, H. Infrared Spectroscopic Analysis of the Inorganic Deposits from Water in Domestic and Technical Heat Exchangers. Energies 2018, 11, 798. [Google Scholar] [CrossRef] [Green Version]
- Klyndyuk, A.; Krasutskaya, N.; Khort, A. Synthesis and Properties of Ceramics Based on a Layered Bismuth Calcium Cobaltite. Inorg. Mater. 2018, 54, 509–514. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, H.; Zhou, X.; Wang, Y.; Zuo, K.; Cheng, H. Thermal Migration Behavior of Na+, Cu2+ and Li+ in Montmorillonite. Minerals 2022, 12, 477. [Google Scholar] [CrossRef]
- Pan, Y.; Shen, X.; Yao, L.; Bentalib, A.; Peng, Z. Active Sites in Heterogeneous Catalytic Reaction on Metal and Metal Oxide: Theory and Practice. Catalysts 2018, 8, 478. [Google Scholar] [CrossRef]
- Parrino, F.; Conte, P.; De Pasquale, C.; Laudicina, V.A.; Loddo, V.; Palmisano, L. Influence of Adsorbed Water on the Activation Energy of Model Photocatalytic Reactions. J. Phys. Chem. C 2017, 121, 2258–2267. [Google Scholar] [CrossRef]
- Rubbens, A.; Drache, M.; Roussel, P.; Wignacourt, J.P. Raman Scattering Characterization of Bismuth Based Mixed Oxides with Bi2O3 Related Structures. Mater. Res. Bull. 2007, 42, 1683–1690. [Google Scholar] [CrossRef]
- Zhang, F.X.; Manoun, B.; Saxena, S.K.; Zha, C.S. Structural Behavior of Sr2Bi2O5 at High Pressures. J. Solid State Chem. 2006, 179, 544–550. [Google Scholar] [CrossRef]
- Roy, D.; Samu, G.F.; Hossain, M.K.; Janáky, C.; Rajeshwar, K. On the Measured Optical Bandgap Values of Inorganic Oxide Semiconductors for Solar Fuels Generation. Catal. Today 2018, 300, 136–144. [Google Scholar] [CrossRef]
- The Materials Project. Materials Data on Ca2Bi2O5 by Materials Project; LBNL Materials Project; Lawrence Berkeley National Lab. (LBNL): Berkeley, CA, USA, 2020. [Google Scholar]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Buongiorno Nardelli, M.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced Capabilities for Materials Modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef]
- Schlipf, M.; Gygi, F. Optimization Algorithm for the Generation of ONCV Pseudopotentials. Comput. Phys. Commun. 2015, 196, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Ye, C.; Gao, H.; Zhang, N.; Zhou, X. Photolysis of Nitric Acid and Nitrate on Natural and Artificial Surfaces. Environ. Sci. Technol. 2016, 50, 3530–3536. [Google Scholar] [CrossRef]
- Chen, L.; Kong, L.; Tong, S.; Yang, K.; Jin, S.; Wang, C.; Wang, L. Aqueous Phase Oxidation of Bisulfite Influenced by Nitrate Photolysis. Sci. Total Environ. 2021, 785, 147345. [Google Scholar] [CrossRef]
- Zafiriou, O.C.; True, M.B. Nitrate Photolysis in Seawater by Sunlight. Mar. Chem. 1979, 8, 33–42. [Google Scholar] [CrossRef]
- Ferrari, C.; Chen, H.; Lavezza, R.; Santinelli, C.; Longo, I.; Bramanti, E. Photodegradation of Rhodamine B Using the Microwave/UV/H2O2: Effect of Temperature. Int. J. Photoenergy 2013, 2013, e854857. [Google Scholar] [CrossRef]
- McHedlov-Petrosyan, N.O.; Kholin, Y.V. Aggregation of Rhodamine B in Water. Russ. J. Appl. Chem. 2004, 77, 414–422. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, J.; Hidaka, H. Highly Selective Deethylation of Rhodamine B: Adsorption and Photooxidation Pathways of the Dye on the TiO2/SiO2 Composite Photocatalyst. Int. J. Photoenergy 2003, 5, 209–217. [Google Scholar] [CrossRef]
- Bahmani, A.; Sellami, M.; Bettahar, N. Synthesis of Bismuth Mixed Oxide by Thermal Decomposition of a Coprecipitate Precursor. J. Therm. Anal. Calorim. 2012, 107, 955–962. [Google Scholar] [CrossRef]
- Huerta-Flores, A.M.; Sánchez-Martínez, D.; del Rocío Hernández-Romero, M.; Zarazúa-Morín, M.E.; Torres-Martínez, L.M. Visible-Light-Driven BaBiO3 Perovskite Photocatalysts: Effect of Physicochemical Properties on the Photoactivity towards Water Splitting and the Removal of Rhodamine B from Aqueous Systems. J. Photochem. Photobiol. Chem. 2019, 368, 70–77. [Google Scholar] [CrossRef]
- Patrocinio, A.O.T.; Schneider, J.; França, M.D.; Santos, L.M.; Caixeta, B.P.; Machado, A.E.H.; Bahnemann, D.W. Charge Carrier Dynamics and Photocatalytic Behavior of TiO2 Nanopowders Submitted to Hydrothermal or Conventional Heat Treatment. RSC Adv. 2015, 5, 70536–70545. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Cai, J.; Luo, M.; Leihong, Z. Photocatalytic Degradation of Methylene Blue on CaBi6O10/Bi2O3 Composites under Visible Light. Chem. Eng. J. 2012, s189–s190, 473–481. [Google Scholar] [CrossRef]
- Shtarev, D.; Doronin, I.; Blokh, A.; Shtareva, A. Dependency of the Optical Properties of Heterogeneous Calcium Bismuthate–Bismuth Oxide Particles on the Order of Layers Alternation. Opt. Quantum Electron. 2016, 48, 1–9. [Google Scholar] [CrossRef]
- Wu, Z.; Zeng, D.; Liu, X.; Yu, C.; Yang, K.; Liu, M. Hierarchical δ-Bi2O3/Bi2O2CO3 Composite Microspheres: Phase Transformation Fabrication, Characterization and High Photocatalytic Performance. Res. Chem. Intermed. 2018, 44, 5995–6010. [Google Scholar] [CrossRef]











| CBO Type | Catalyst Concentration (ppm) | Contaminant Type | Volume (mL) and Concentration (ppm) of Contaminant | Degradation Efficiency of Contaminant (%) | Irradiation Source | Source |
|---|---|---|---|---|---|---|
| CaBi6O10/Bi2O3 | 10 | Methylene blue | 300 mL of 1 ppm | 97% after 180 minutes of irradiation and 1 h of adsorption | Visible light irradiation | [16] |
| CaBi6O10 | 10 | Methylene blue | 300 mL of 1 ppm | 75% after 180 minutes of irradiation and 1 h of adsorption | Visible light irradiation | [16] |
| CaBiO3 | 300 | Ciprofloxacin | 100 mL of 10 ppm | 90.5% after 90 minutes of irradiation and 15 minutes of adsorption | Solar light irradiation | [12] |
| CaBiO3 | 300 | Tetracycline | 100 mL of 30 ppm | 68.6% after 90 minutes of irradiation and 15 minutes of adsorption | Solar light irradiation | [12] |
| CaBi2O4−x | 1500 | Methylene blue | 100 mL of 16 ppm | 100% after 75 minutes of irradiation and 30 minutes of adsorption | Visible light irradiation | [20] |
| Ca2Bi2O5 | 167 | Methylene blue | 300 mL of 10 ppm | 87% after 40 minutes of irradiation and 30 minutes of adsorption | Visible light irradiation | [21] |
| Ca2Bi2O5 | 167 | Rhodamine B | 300 mL of 10 ppm | 93% after 120 minutes of irradiation and 30 minutes of adsorption | Visible light irradiation | [22] |
| Ca2Bi2O5/0.1α-Bi2O3 | 167 | Rhodamine B | 300 mL of 10 ppm | 100% after 60 minutes of irradiation and 30 minutes of adsorption | Visible light irradiation | [22] |
| CaBiO3 | 900 | Cr(VI) | 100 mL of 364 ppm | 94% after 120 minutes of irradiation and 45 minutes of adsorption | Visible light irradiation | [13] |
| EDAX ZAF Quantification (Standardless) | |||
|---|---|---|---|
| Element Normalized | Wt % | At % | |
| O K | 14.29 | 56.43 | |
| CaK | 13.87 | 21.86 | |
| BiL | 71.83 | 21.71 | |
| ICP | |||
| Element | Molar Ratio | ||
| Ca | 1 | ||
| Bi | 1 | ||
| Catalyst | Fitting Parameters (First Order Reaction) | |
|---|---|---|
| k (min−1) | R2 | |
| pH = 5 | ||
| CBO-600 | 0.0027 | 0.9999 |
| CBO-725 | 0.0036 | 0.9978 |
| pH = 2 | ||
| CBO-725 | 0.0053 | 0.9830 |
| CBO-775 | 0.0069 | 0.9973 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadiș, A.I.; Rus, F.Ș.; Gonçalves, J.N.; Ivanovici, M. Preparing a Ca-Bi-O System by the Precipitation Method and Studying Its Intermediate Structural Properties for Applications in Water Treatment. Inorganics 2023, 11, 79. https://doi.org/10.3390/inorganics11020079
Cadiș AI, Rus FȘ, Gonçalves JN, Ivanovici M. Preparing a Ca-Bi-O System by the Precipitation Method and Studying Its Intermediate Structural Properties for Applications in Water Treatment. Inorganics. 2023; 11(2):79. https://doi.org/10.3390/inorganics11020079
Chicago/Turabian StyleCadiș, Adrian Ionuț, Florina Ștefania Rus, João Nuno Gonçalves, and Mădălina Ivanovici. 2023. "Preparing a Ca-Bi-O System by the Precipitation Method and Studying Its Intermediate Structural Properties for Applications in Water Treatment" Inorganics 11, no. 2: 79. https://doi.org/10.3390/inorganics11020079
APA StyleCadiș, A. I., Rus, F. Ș., Gonçalves, J. N., & Ivanovici, M. (2023). Preparing a Ca-Bi-O System by the Precipitation Method and Studying Its Intermediate Structural Properties for Applications in Water Treatment. Inorganics, 11(2), 79. https://doi.org/10.3390/inorganics11020079