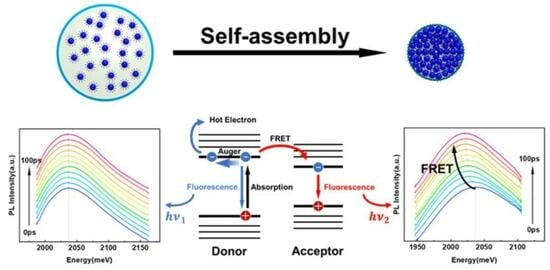
Fluorescence Resonance Energy Transfer Properties and Auger Recombination Suppression in Supraparticles Self-Assembled from Colloidal Quantum Dots
Abstract
:1. Introduction
2. Self-Assembly of CQDs into Supraparticles
3. Structural Characterizations of Supraparticles
4. Single Supraparticle Spectroscopy and Analysis
5. Suppression of Auger Recombination
6. Materials and Methods
6.1. Synthesis of Supraparticles Structures
6.2. Structural Characterization
6.3. Optical Characterization
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barak, Y.; Meir, I.; Shapiro, A.; Jang, Y.; Lifshitz, E. Fundamental Properties in Colloidal Quantum Dots. Adv. Mater. 2018, 30, e1801442. [Google Scholar] [CrossRef] [PubMed]
- Chen, O.; Zhao, J.; Chauhan, V.P.; Cui, J.; Wong, C.; Harris, D.K.; Wei, H.; Han, H.-S.; Fukumura, D.; Jain, R.K.; et al. Compact high-quality CdSe-CdS core-shell nanocrystals with narrow emission linewidths and suppressed blinking. Nat. Mater. 2013, 12, 445–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.; Alivisatos, A.P. Colloidal nanocrystal synthesis and the organic-inorganic interface. Nature 2005, 437, 664–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Chen, Y.; Tan, C.-S.; Quintero-Bermudez, R.; Proppe, A.H.; Munir, R.; Tan, H.; Voznyy, O.; Scheffel, B.; Walters, G.; et al. Lattice anchoring stabilizes solution-processed semiconductors. Nature 2019, 570, 96–101. [Google Scholar] [CrossRef]
- Cargnello, M.; Johnston-Peck, A.C.; Diroll, B.T.; Wong, E.; Datta, B.; Damodhar, D.; Doan-Nguyen, V.V.T.; Herzing, A.A.; Kagan, C.R.; Murray, C.B. Substitutional doping in nanocrystal superlattices. Nature 2015, 524, 450–453. [Google Scholar] [CrossRef]
- Peng, X.; Lv, L.; Liu, S.; Li, J.; Lei, H.; Qin, H. Synthesis of Weakly Confined, Cube-Shaped, and Monodisperse Cadmium Chalcogenide Nanocrystals with Unexpected Photophysical Properties. J. Am. Chem. Soc. 2022, 144, 16872–16882. [Google Scholar] [CrossRef]
- Shirasaki, Y.; Supran, G.J.; Bawendi, M.G.; Bulovic, V. Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photonics 2013, 7, 13–23. [Google Scholar] [CrossRef]
- Chang, H.; Dong, H.; Zhao, J.; Zhang, L. Efficient and stable solid state luminophores with colloidal quantum dots-based silica monolith. Solid State Commun. 2020, 305, 113765. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, Y.; Cao, W.; Titov, A.; Hyvonen, J.; Manders, J.R.; Xue, J.; Holloway, P.H.; Qian, L. High-efficiency light-emitting devices based on quantum dots with tailored nanostructures. Nat. Photonics 2015, 9, 259–266. [Google Scholar] [CrossRef]
- Kagan, C.R.; Lifshitz, E.; Sargent, E.H.; Talapin, D.V. Building devices from colloidal quantum dots. Science 2016, 353, aac5523. [Google Scholar] [CrossRef]
- Zhao, B.; Yao, Y.; Gao, M.; Sun, K.; Zhang, J.; Li, W. Doped quantum dot@silica nanocomposites for white light-emitting diodes. Nanoscale 2015, 7, 17231–17236. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot-induced phase stabilization of alpha-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robel, I.; Subramanian, V.; Kuno, M.; Kamat, P.V. Quantum dot solar cells. Harvesting light energy with CdSe nanocrystals molecularly linked to mesoscopic TiO2 films. J. Am. Chem. Soc. 2006, 128, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Cooney, R.R.; Sewall, S.L.; Sagar, D.M.; Kambhampati, P. Gain Control in Semiconductor Quantum Dots via State-Resolved Optical Pumping. Phys. Rev. Lett. 2009, 102, 127404. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, D.; Wang, Z.; Li, X.; Chen, X.; Nalla, V.; Zeng, H.; Sun, H. Solution-Grown CsPbBr3/Cs4PbBr6 Perovskite Nanocomposites: Toward Temperature-Insensitive Optical Gain. Small 2017, 13, 1587. [Google Scholar]
- Park, Y.-S.; Roh, J.; Diroll, B.T.; Schaller, R.D.; Klimov, V.I. Colloidal quantum dot lasers. Nat. Rev. Mater. 2021, 6, 382–401. [Google Scholar] [CrossRef]
- Taghipour, N.; Dalmases, M.; Whitworth, G.L.; Dosil, M.; Othonos, A.; Christodoulou, S.; Liga, S.M.; Konstantatos, G. Colloidal Quantum Dot Infrared Lasers Featuring Sub-Single-Exciton Threshold and Very High Gain. Adv. Mater. 2023, 35, 2207678. [Google Scholar] [CrossRef]
- Chen, O.; Riedemann, L.; Etoc, F.; Herrmann, H.; Coppey, M.; Barch, M.; Farrar, C.T.; Zhao, J.; Bruns, O.T.; Wei, H.; et al. Magneto-fluorescent core-shell supernanoparticles. Nat. Commun. 2014, 5, 5093. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Lu, Z.; Li, Z.; Yin, Y. Assembly of Colloidal Nanoparticles into Hollow Superstructures by Controlling Phase Separation in Emulsion Droplets. Small Struct. 2021, 2, 2100005. [Google Scholar] [CrossRef]
- Kim, K.-H.; Dannenberg, P.H.; Yan, H.; Cho, S.; Yun, S.-H. Compact Quantum-Dot Microbeads with Sub-Nanometer Emission Linewidth. Adv. Funct. Mater. 2021, 31, 2103413. [Google Scholar] [CrossRef]
- He, X.; Jia, K.; Bai, Y.; Chen, Z.; Liu, Y.; Huang, Y.; Liu, X. Quantum dots encoded white-emitting polymeric superparticles for simultaneous detection of multiple heavy metal ions. J. Hazard. Mater. 2021, 405, 124263. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Ohshiro, K.; Watanabe, T.; Hyeon-Deuk, K.; Kim, D. Temperature-Dependent Exciton Dynamics in CdTe Quantum Dot Superlattices Fabricated via Layer-by-Layer Assembly. Adv. Opt. Mater. 2022, 10, 2102781. [Google Scholar] [CrossRef]
- Lan, X.; Chen, M.; Hudson, M.H.; Kamysbayev, V.; Wang, Y.; Guyot-Sionnest, P.; Talapin, D.V. Quantum dot solids showing state-resolved band-like transport. Nat. Mater. 2020, 19, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Crisp, R.W.; Schrauben, J.N.; Beard, M.C.; Luther, J.M.; Johnson, J.C. Coherent Exciton Delocalization in Strongly Coupled Quantum Dot Arrays. Nano Lett. 2013, 13, 4862–4869. [Google Scholar] [CrossRef]
- Hou, K.; Han, J.; Tang, Z. Formation of Supraparticles and Their Application in Catalysis. ACS Mater. Lett. 2020, 2, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Li, R.; Guo, G.; Xia, Y. Janus and core@shell gold nanorod@Cu2-xS supraparticles: Reactive site regulation fabrication, optical/catalytic synergetic effects and enhanced photothermal efficiency/photostability. Chem. Commun. 2020, 56, 8996–8999. [Google Scholar] [CrossRef]
- Lin, C.H.; Lafalce, E.; Jung, J.; Smith, M.J.; Malak, S.T.; Aryal, S.; Yoon, Y.J.; Zhai, Y.; Lin, Z.; Vardeny, Z.V.; et al. Core/Alloyed-Shell Quantum Dot Robust Solid Films with High Optical Gains. ACS Photonics 2016, 3, 647–658. [Google Scholar] [CrossRef]
- Gur, I.; Fromer, N.A.; Geier, M.L.; Alivisatos, A.P. Air-stable all-inorganic nanocrystal solar cells processed from solution. Science 2005, 310, 462–465. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.; Wang, P.; Li, X.; Hu, X.; Hou, J.; Wang, L.; Zhang, F. Near-Infrared-Triggered Azobenzene-Liposome/Upconversion Nanoparticle Hybrid Vesicles for Remotely Controlled Drug Delivery to Overcome Cancer Multidrug Resistance. Adv. Mater. 2016, 28, 9341–9348. [Google Scholar] [CrossRef]
- Klimov, V.I.; Mikhailovsky, A.A.; McBranch, D.W.; Leatherdale, C.A.; Bawendi, M.G. Quantization of multiparticle Auger rates in semiconductor quantum dots. Science 2000, 287, 1011–1013. [Google Scholar] [CrossRef]
- Talapin, D.V.; Lee, J.-S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of Colloidal Nanocrystals for Electronic and Optoelectronic Applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shang, L.; Cheng, Y.; Gu, Z. Spherical Colloidal Photonic Crystals. Acc. Chem. Res. 2014, 47, 3632–3642. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-G.; Kim, S.-H.; Magkiriadou, S.; Choi, T.M.; Kim, Y.-S.; Manoharan, V.N. Full-Spectrum Photonic Pigments with Non-iridescent Structural Colors through Colloidal Assembly. Angew. Chem. Int. Ed. 2014, 53, 2899–2903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, N.; Utech, S.; England, G.T.; Shirman, T.; Phillips, K.R.; Koay, N.; Burgess, I.B.; Kolle, M.; Weitz, D.A.; Aizenberg, J. Color from hierarchy: Diverse optical properties of micron-sized spherical colloidal assemblies. Proc. Natl. Acad. Sci. USA 2015, 112, 10845–10850. [Google Scholar] [CrossRef] [Green Version]
- Marino, E.; Sciortino, A.; Berkhout, A.; MacArthur, K.E.; Heggen, M.; Gregorkiewicz, T.; Kodger, T.E.; Capretti, A.; Murray, C.B.; Koenderink, A.F.; et al. Simultaneous Photonic and Excitonic Coupling in Spherical Quantum Dot Supercrystals. ACS Nano 2020, 14, 13806–13815. [Google Scholar] [CrossRef]
- Chen, X.; Fu, Z.; Gong, Q.; Wang, J. Quantum entanglement on photonic chips: A review. Adv. Photonics 2021, 3, 064002. [Google Scholar] [CrossRef]
- Jiang, B.; Zhu, S.; Ren, L.; Shi, L.; Zhang, X. Simultaneous ultraviolet, visible, and near-infrared continuous-wave lasing in a rare-earth-doped microcavity. Adv. Photonics 2022, 4, 046003. [Google Scholar] [CrossRef]
- Shuklov, I.A.; Toknova, V.F.; Lizunova, A.A.; Razumov, V.F. Controlled aging of PbS colloidal quantum dots under mild conditions. Mater. Today Chem. 2020, 18, 100357. [Google Scholar] [CrossRef]
- Wu, J.; Su, R.; Fieramosca, A.; Ghosh, S.; Zhao, J.; Liew, T.C.H.; Xiong, Q. Perovskite polariton parametric oscillator. Adv. Photonics 2021, 3, 055003. [Google Scholar] [CrossRef]
- Bai, F.; Wang, D.; Huo, Z.; Chen, W.; Liu, L.; Liang, X.; Chen, C.; Wang, X.; Peng, Q.; Li, Y. A versatile bottom-up assembly approach to colloidal spheres from nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 6650–6653. [Google Scholar] [CrossRef]
- De Nijs, B.; Dussi, S.; Smallenburg, F.; Meeldijk, J.D.; Groenendijk, D.J.; Filion, L.; Imhof, A.; van Blaaderen, A.; Dijkstra, M. Entropy-driven formation of large icosahedral colloidal clusters by spherical confinement. Nat. Mater. 2015, 14, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Lacava, J.; Ouali, A.-A.; Raillard, B.; Kraus, T. On the behaviour of nanoparticles in oil-in-water emulsions with different surfactants. Soft Matter 2014, 10, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, A.; Eldridge, C.; Schneider, G.A.; Domenech, B. Controlling the Large-Scale Fabrication of Supraparticles. J. Phys. Chem. B 2020, 124, 11263–11272. [Google Scholar] [CrossRef] [PubMed]
- Koshkina, O.; Raju, L.T.; Kaltbeitzel, A.; Riedinger, A.; Lohse, D.; Zhang, X.; Landfester, K. Surface Properties of Colloidal Particles Affect Colloidal Self-Assembly in Evaporating Self-Lubricating Ternary Droplets. ACS Appl. Mater. Interfaces 2022, 14, 2275–2290. [Google Scholar] [CrossRef]
- Marino, E.; Kodger, T.E.; Wegdam, G.H.; Schall, P. Revealing Driving Forces in Quantum Dot Supercrystal Assembly. Adv. Mater. 2018, 30, 1803433. [Google Scholar] [CrossRef] [Green Version]
- Montanarella, F.; Geuchies, J.J.; Dasgupta, T.; Prins, P.T.; van Overbeek, C.; Dattani, R.; Baesjou, P.; Dijkstra, M.; Petukhov, A.V.; van Blaaderen, A.; et al. Crystallization of Nanocrystals in Spherical Confinement Probed by in Situ X-ray Scattering. Nano Lett. 2018, 18, 3675–3681. [Google Scholar] [CrossRef]
- Schmitt, J.; Hajiw, S.; Lecchi, A.; Degrouard, J.; Salonen, A.; Imperor-Clerc, M.; Pansu, B. Formation of Superlattices of Gold Nanoparticles Using Ostwald Ripening in Emulsions: Transition from fcc to bcc Structure. J. Phys. Chem. B 2016, 120, 5759–5766. [Google Scholar] [CrossRef] [Green Version]
- Marino, E.; Keller, A.W.; An, D.; van Dongen, S.; Kodger, T.E.; MacArthur, K.E.; Heggen, M.; Kagan, C.R.; Murray, C.B.; Schall, P. Favoring the Growth of High-Quality, Three-Dimensional Supercrystals of Nanocrystals. J. Phys. Chem. C 2020, 124, 11256–11264. [Google Scholar] [CrossRef]
- Wang, D.; van der Wee, E.B.; Zanaga, D.; Altantzis, T.; Wu, Y.; Dasgupta, T.; Dijkstra, M.; Murray, C.B.; Bals, S.; van Blaaderen, A. Quantitative 3D real-space analysis of Laves phase supraparticles. Nat. Commun. 2021, 12, 3980. [Google Scholar] [CrossRef]
- Montanarella, F.; Altantzis, T.; Zanaga, D.; Rabouw, F.T.; Bals, S.; Baesjou, P.; Vanmaekelbergh, D.; van Blaaderen, A. Composite Supraparticles with Tunable Light Emission. ACS Nano 2017, 11, 9136–9142. [Google Scholar] [CrossRef] [Green Version]
- Zou, H.; Wang, D.; Gong, B.; Liu, Y. Preparation of CdTe superparticles for white light-emitting diodes without Forster resonance energy transfer. RSC Adv. 2019, 9, 30797–30802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanmaekelbergh, D.; van Vugt, L.K.; Bakker, H.E.; Rabouw, F.T.; de Nijs, B.; van Dijk-Moes, R.J.A.; van Huis, M.A.; Baesjou, P.J.; van Blaaderen, A. Shape-Dependent Multiexciton Emission and Whispering Gallery Modes in Supraparticles of CdSe/Multishell Quantum Dots. ACS Nano 2015, 9, 3942–3950. [Google Scholar] [CrossRef] [PubMed]
- Montanarella, F.; Urbonas, D.; Chadwick, L.; Moerman, P.G.; Baesjou, P.J.; Mahrt, R.F.; van Blaaderen, A.; Stoferle, T.; Vanmaekelbergh, D. Lasing Supraparticles Self-Assembled from Nanocrystals. ACS Nano 2018, 12, 12788–12794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuhaus, S.J.; Marino, E.; Murray, C.B.; Kagan, C.R. Frequency Stabilization and Optically Tunable Lasing in Colloidal Quantum Dot Superparticles. Nano Lett. 2023, 23, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zhong, Y.; Dong, H.; Wang, Z.; Xie, W.; Pan, A.; Zhang, L. Ultrastable low-cost colloidal quantum dot microlasers of operative temperature up to 450 K. Light Sci. Appl. 2021, 10, 60. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, W.; Yan, Y.; Yi, J.; Dong, H.; Wang, K.; Yao, J.; Zhao, Y.S. Proton-Controlled Organic Microlaser Switch. ACS Nano 2018, 12, 5734–5740. [Google Scholar] [CrossRef]
- Blondot, V.; Bogicevic, A.; Coste, A.; Arnold, C.; Buil, S.; Quelin, X.; Pons, T.; Lequeux, N.; Hermier, J.-P. Fluorescence properties of self assembled colloidal supraparticles from CdSe/CdS/ZnS nanocrystals. New J. Phys. 2020, 22, 113026. [Google Scholar] [CrossRef]
- Montanarella, F.; Biondi, M.; Hinterding, S.M.; Vanmaekelbergh, D.; Rabouw, F.T. Reversible Charge-Carrier Trapping Slows Forster Energy Transfer in CdSe/CdS Quantum-Dot Solids. Nano Lett. 2018, 18, 5867–5874. [Google Scholar] [CrossRef] [Green Version]
- Kister, T.; Mravlak, M.; Schilling, T.; Kraus, T. Pressure-controlled formation of crystalline, Janus, and core-shell supraparticles. Nanoscale 2016, 8, 13377–13384. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Mbah, C.F.; Przybilla, T.; Zubiri, B.A.; Spiecker, E.; Engel, M.; Vogel, N. Magic number colloidal clusters as minimum free energy structures. Nat. Commun. 2018, 9, 5259. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Jung, K.; Yu, J.W.; Park, S.; Kim, S.-H.; Lee, W.B.; Hwang, H.; Manoharan, V.N.; Moon, J.H. Controlled Assembly of Icosahedral Colloidal Clusters for Structural Coloration. Chem. Mater. 2020, 32, 9704–9712. [Google Scholar] [CrossRef]
- Chou, K.F.; Dennis, A.M. Forster Resonance Energy Transfer between Quantum Dot Donors and Quantum Dot Acceptors. Sensors 2015, 15, 13288–13325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clapp, A.R.; Medintz, I.L.; Mauro, J.M.; Fisher, B.R.; Bawendi, M.G.; Mattoussi, H. Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors. J. Am. Chem. Soc. 2004, 126, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Kang, J.; Qin, H.; Chen, X.; Ma, J.; Zhou, J.; Chen, L.; Wang, L.; Wang, L.-W.; Peng, X. Engineering Auger recombination in colloidal quantum dots via dielectric screening. Nat. Commun. 2019, 10, 1750. [Google Scholar] [CrossRef] [Green Version]
- Vaxenburg, R.; Rodina, A.; Shabaev, A.; Lifshitz, E.; Efros, A.L. Nonradiative Auger Recombination in Semiconductor Nanocrystals. Nano Lett. 2015, 15, 2092–2098. [Google Scholar] [CrossRef]
- Javaux, C.; Mahler, B.; Dubertret, B.; Shabaev, A.; Rodina, A.V.; Efros, A.L.; Yakovlev, D.R.; Liu, F.; Bayer, M.; Camps, G.; et al. Thermal activation of non-radiative Auger recombination in charged colloidal nanocrystals. Nat. Nanotechnol. 2013, 8, 206–212. [Google Scholar] [CrossRef]
- Algar, W.R.; Kim, H.; Medintz, I.L.; Hildebrandt, N. Emerging non-traditional Forster resonance energy transfer configurations with semiconductor quantum dots: Investigations and applications. Coord. Chem. Rev. 2014, 263, 65–85. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Chang, H.; Dong, H.; Zhang, C.; Zhang, L. Fluorescence Resonance Energy Transfer Properties and Auger Recombination Suppression in Supraparticles Self-Assembled from Colloidal Quantum Dots. Inorganics 2023, 11, 218. https://doi.org/10.3390/inorganics11050218
Tian X, Chang H, Dong H, Zhang C, Zhang L. Fluorescence Resonance Energy Transfer Properties and Auger Recombination Suppression in Supraparticles Self-Assembled from Colloidal Quantum Dots. Inorganics. 2023; 11(5):218. https://doi.org/10.3390/inorganics11050218
Chicago/Turabian StyleTian, Xinhua, Hao Chang, Hongxing Dong, Chi Zhang, and Long Zhang. 2023. "Fluorescence Resonance Energy Transfer Properties and Auger Recombination Suppression in Supraparticles Self-Assembled from Colloidal Quantum Dots" Inorganics 11, no. 5: 218. https://doi.org/10.3390/inorganics11050218
APA StyleTian, X., Chang, H., Dong, H., Zhang, C., & Zhang, L. (2023). Fluorescence Resonance Energy Transfer Properties and Auger Recombination Suppression in Supraparticles Self-Assembled from Colloidal Quantum Dots. Inorganics, 11(5), 218. https://doi.org/10.3390/inorganics11050218