Toward High-Performances of Halide Light-Emitting Diodes: The Importance of Ligands Engineering
Abstract
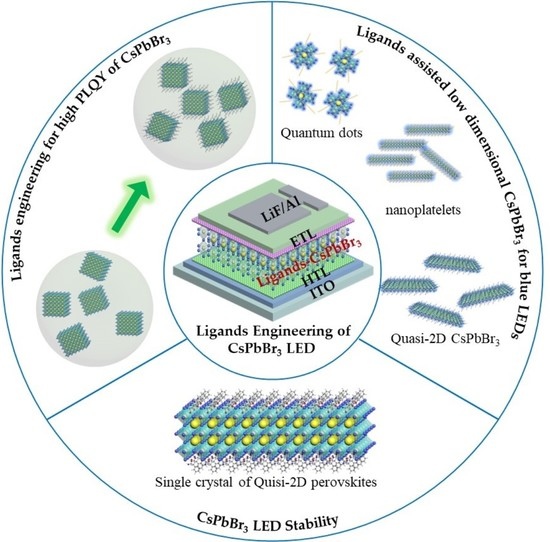
:1. Introduction
2. Toward High Radiative Recombination of CsPbBr3
3. Blue Emission of LEDs
4. Stabilities of Materials and Devices
5. Perspectives and Summary
Funding
Conflicts of Interest
References
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M.V.; Manna, L. Genesis, Challenges and Opportunities for Colloidal Lead Halide Perovskite Nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, Y.; Ruan, C.; Yin, C.; Wang, X.; Wang, Y.; Yu, W.W. Efficient and Stable White LEDs with Silica-Coated Inorganic Perovskite Quantum Dots. Adv. Mater. 2016, 28, 10088–10094. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jiang, Y.; Jiang, Y.; Guo, Y.; Liu, Y.; Nakamura, E. Chemical Formation and Multiple Applications of Organic–Inorganic Hybrid Perovskite Materials. J. Am. Chem. Soc. 2019, 141, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Hsu, B.; Chen, C.; Lee, C.; Liu, H.; Wang, H.; Huang, Y.; Wu, T.; Manikandan, A.; Ho, R.; et al. Perovskite Quantum Dots with Near Unity Solution and Neat-Film Photoluminescent Quantum Yield by Novel Spray Synthesis. Adv. Mater. 2018, 30, 1705532. [Google Scholar] [CrossRef]
- Minh, D.N.; Kim, J.; Hyon, J.; Sim, J.H.; Sowlih, H.H.; Seo, C.; Nam, J.; Eom, S.; Suk, S.; Lee, S.; et al. Room-Temperature Synthesis of Widely Tunable Formamidinium Lead Halide Perovskite Nanocrystals. Chem. Mater. 2017, 29, 5713–5719. [Google Scholar] [CrossRef]
- Chen, Q.; De Marco, N.; Yang, Y.; Song, T.-B.; Chen, C.-C.; Zhao, H.; Hong, Z.; Zhou, H.; Yang, Y. Under the Spotlight: The Organic–Inorganic Hybrid Halide Perovskite for Optoelectronic Applications. Nano Today 2015, 10, 355–396. [Google Scholar] [CrossRef]
- Ding, J.; Yan, Q. Progress in Organic-Inorganic Hybrid Halide Perovskite Single Crystal: Growth Techniques and Applications. Sci. China Mater. 2017, 60, 1063–1078. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Su, F.; Tan, J.; Luo, Y.; Ye, S. Conformational Disorder of Organic Cations Tunes the Charge Carrier Mobility in Two-Dimensional Organic-Inorganic Perovskites. Nat. Commun. 2020, 11, 5481–5489. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Hawash, Z.; Raga, S.R.; Ono, L.K.; Qi, Y. Thermal Degradation of CH3NH3PbI3 Perovskite into NH3 and CH3I Gases Observed by Coupled Thermogravimetry–Mass Spectrometry Analysis. Energy Environ. Sci. 2016, 9, 3406–3410. [Google Scholar] [CrossRef]
- Xiao, Z.; Song, Z.; Yan, Y. From Lead Halide Perovskites to Lead-Free Metal Halide Perovskites and Perovskite Derivatives. Adv. Mater. 2019, 31, 1803792. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Tong, G.; Cao, Y.; Tang, M.; Xu, J.; Yu, L.; Chen, K. The Effect of Decomposed PbI2 on Microscopic Mechanisms of Scattering in CH3NH3PbI3 Films. Nanoscale Res. Lett. 2019, 14, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fang, Z.; Guo, T.; Zhao, R.; Deng, Z.; Zhang, J.; Shang, M.; Liu, X.; Liu, J.; Huang, L.; et al. Robust Heterojunction to Strengthen the Performances of FAPbI3 Perovskite Solar Cells. Chem. Eng. J. 2022, 432, 134311. [Google Scholar] [CrossRef]
- Yan, F.; Tan, S.T.; Li, X.; Demir, H.V. Light Generation in Lead Halide Perovskite Nanocrystals: LEDs, Color Converters, Lasers, and Other Applications. Small 2019, 15, 1902079. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Guo, J.; Sun, S.; Lu, P.; Wu, J.; Wang, Y.; Kershaw, S.V.; Yu, W.W.; Rogach, A.L.; Zhang, Y. Bright CsPbI3 Perovskite Quantum Dot Light-Emitting Diodes with Top-Emitting Structure and a Low Efficiency Roll-Off Realized by Applying Zirconium Acetylacetonate Surface Modification. Nano Lett. 2020, 20, 2829–2836. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Li, X.; Yuan, M.; Turyanska, L.; Yang, X. Core/Shell Perovskite Nanocrystals: Synthesis of Highly Efficient and Environmentally Stable FAPbBr3/CsPbBr3 for LED Applications. Adv. Funct. Mater. 2020, 30, 1910582. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, Z.; Lin, J. An Overview on Enhancing the Stability of Lead Halide Perovskite Quantum Dots and Their Applications in Phosphor-Converted LEDs. Chem. Soc. Rev. 2019, 48, 310–350. [Google Scholar] [CrossRef]
- Xie, K.; Wei, S.; Alhadhrami, A.; Liu, J.; Zhang, P.; Elnaggar, A.Y.; Zhang, F.; Mahmoud, M.H.H.; Murugadoss, V.; El-Bahy, S.M.; et al. Synthesis of CsPbBr3/CsPb2Br5@Silica Yolk-Shell Composite Microspheres: Precisely Controllable Structure and Improved Catalytic Activity for Dye Degradation. Adv. Compos. Hybrid Mater. 2022, 5, 1423–1432. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Z.; Zhang, F.; Zhao, M.; Li, C.; Li, J.; Wen, B.; Wang, F. In Situ Growth of Lead-Free Halide Perovskites into SiO2 Sub-Microcapsules Toward Water-Stable Photocatalytic CO2 Reduction. Nanoscale 2023, 15, 7023–7031. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Wang, W.; Zhang, J.; Xu, B.; Karen, K.L.; Zheng, Y.; Liu, S.; Chen, S.; Wang, K.; et al. Hybrid Perovskite Light-Emitting Diodes Based on Perovskite Nanocrystals with Organic-Inorganic Mixed Cations. Adv. Mater. 2017, 29, 1606405. [Google Scholar] [CrossRef]
- Amgar, D.; Binyamin, T.; Uvarov, V.; Etgar, L. Near Ultra-Violet to Mid-Visible Band Gap Tuning of Mixed Cation RbxCs1−xPbX3 (X = Cl or Br) Perovskite Nanoparticles. Nanoscale 2018, 10, 6060–6068. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, B.; Zhang, Q.; Li, Z.; Shan, A.; Li, L. Postsynthesis Potassium-Modification Method to Improve Stability of CsPbBr3 Perovskite Nanocrystals. Adv. Opt. Mater. 2018, 6, 1701106. [Google Scholar] [CrossRef]
- Tong, G.; Ono, L.K.; Qi, Y. Recent Progress of All-Bromide Inorganic Perovskite Solar Cells. Energy Technol.-Ger. 2020, 8, 1900961. [Google Scholar] [CrossRef]
- Nenon, D.P.; Pressler, K.; Kang, J.; Koscher, B.A.; Olshansky, J.H.; Osowiecki, W.T.; Koc, M.A.; Wang, L.-W.; Alivisatos, A.P. Design Principles for Trap-Free CsPbX3 Nanocrystals: Enumerating and Eliminating Surface Halide Vacancies with Softer Lewis Bases. J. Am. Chem. Soc. 2018, 140, 17760–17772. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, Y.; Zeng, S.; Cui, Y.; Xiao, Y. Surface Regulation of CsPbBr3 Quantum Dots for Standard Blue-Emission with Boosted PLQY. Adv. Opt. Mater. 2020, 8, 2000167. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 Quantum Dots for Lighting and Displays: Room-Temperature Synthesis, Photoluminescence Superiorities, Underlying Origins and White Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Shamsi, J.; Kubicki, D.; Anaya, M.; Liu, Y.; Ji, K.; Frohna, K.; Grey, C.P.; Friend, R.H.; Stranks, S.D. Stable Hexylphosphonate-Capped Blue-Emitting Quantum-Confined CsPbBr3 Nanoplatelets. ACS Energy Lett. 2020, 5, 1900–1907. [Google Scholar] [CrossRef]
- Yuan, L.; Li, D.; Liu, H.; Zhang, F.; Wang, S. Quantum-Confined Dodecahedron CsPbBr3 Quantum Dots by A Sequential Post-Treatment Strategy for Efficient Blue PeLEDs. Adv. Funct. Mater. 2022, 32, 2208065. [Google Scholar] [CrossRef]
- Li, Y.; Huang, H.; Xiong, Y.; Kershaw, S.V.; Rogach, A.L. Reversible Transformation Between CsPbBr3 and Cs4PbBr6 Nanocrystals. CrystEngComm 2018, 20, 4900–4904. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Y.; Bekenstein, Y.; Yu, Y.; Gibson, N.A.; Wong, A.B.; Eaton, S.W.; Kornienko, N.; Kong, Q.; Lai, M.; et al. Synthesis of Composition Tunable and Highly Luminescent Cesium Lead Halide Nanowires through Anion-Exchange Reactions. J. Am. Chem. Soc. 2016, 138, 7236–7241. [Google Scholar] [CrossRef]
- Ullah, S.; Wang, J.; Yang, P.; Liu, L.; Yang, S.-E.; Xia, T.; Guo, H.; Chen, Y. All-inorganic CsPbBr3 Perovskite: A Promising Choice for Photovoltaics. Mater. Adv. 2021, 2, 646–683. [Google Scholar] [CrossRef]
- Swarnkar, A.; Mir, W.J.; Nag, A. Can B-Site Doping or Alloying Improve Thermal- and Phase-Stability of All-Inorganic CsPbX3 (X = Cl, Br, I) Perovskites? ACS Energy Lett. 2018, 3, 286–289. [Google Scholar] [CrossRef]
- Hirotsu, S.; Harada, J.; Iizumi, M.; Gesi, K. Structural Phase Transitions in CsPbBr3. J. Phys. Soc. Jpn. 1974, 37, 1393–1398. [Google Scholar] [CrossRef]
- Akbali, B.; Topcu, G.; Guner, T.; Ozcan, M.; Demir, M.M.; Sahin, H. CsPbBr3 perovskites: Theoretical and Experimental Investigation on Water-Assisted Transition from Nanowire Formation to Degradation. Phys. Rev. Mater. 2018, 2, 034601. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Peters, J.A.; Liu, Z.; Sebastian, M.; Im, J.; Chasapis, T.C.; Wibowo, A.C.; Chung, D.Y.; Freeman, A.J.; et al. Crystal Growth of the Perovskite Semiconductor CsPbBr3: A New Material for High-Energy Radiation Detection. Cryst. Growth Des. 2013, 13, 2722–2727. [Google Scholar] [CrossRef]
- Sutton, R.J.; Eperon, G.E.; Miranda, L.; Parrott, E.S.; Kamino, B.A.; Patel, J.B.; Hörantner, M.T.; Johnston, M.B.; Haghighirad, A.A.; Moore, D.T.; et al. Bandgap-Tunable Cesium Lead Halide Perovskites with High Thermal Stability for Efficient Solar Cells. Adv. Energy Mater. 2016, 6, 1502458. [Google Scholar] [CrossRef]
- Ghaithan, H.M.; Alahmed, Z.A.; Qaid, S.M.H.; Hezam, M.; Aldwayyan, A.S. Density Functional Study of Cubic, Tetragonal, and Orthorhombic CsPbBr3 Perovskite. ACS Omega 2020, 5, 7468–7480. [Google Scholar] [CrossRef]
- Maes, J.; Balcaen, L.; Drijvers, E.; Zhao, Q.; De Roo, J.; Vantomme, A.; Vanhaecke, F.; Geiregat, P.; Hens, Z. Light Absorption Coefficient of CsPbBr3 Perovskite Nanocrystals. J. Phys. Chem. Lett. 2018, 9, 3093–3097. [Google Scholar] [CrossRef]
- Yettapu, G.R.; Talukdar, D.; Sarkar, S.; Swarnkar, A.; Nag, A.; Ghosh, P.; Mandal, P. Terahertz Conductivity within Colloidal CsPbBr3 Perovskite Nanocrystals: Remarkably High Carrier Mobilities and Large Diffusion Lengths. Nano Lett. 2016, 16, 4838–4848. [Google Scholar] [CrossRef]
- Kang, Y.; Han, S. Intrinsic Carrier Mobility of Cesium Lead Halide Perovskites. Phys. Rev. Appl. 2018, 10, 044013. [Google Scholar] [CrossRef]
- Song, J.; Cui, Q.; Li, J.; Xu, J.; Wang, Y.; Xu, L.; Xue, J.; Dong, Y.; Tian, T.; Sun, H.; et al. Ultralarge All-Inorganic Perovskite Bulk Single Crystal for High-Performance Visible–Infrared Dual-Modal Photodetectors. Adv. Opt. Mater. 2017, 5, 1700157. [Google Scholar] [CrossRef]
- Lin, K.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite Light-Emitting Diodes with External Quantum Efficiency Exceeding 20 Per Cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Zheng, W.; Zou, C.; Carulli, F.; Zhang, C.; Song, H.; Liu, M.; Zhang, Q.; Lin, L.Y.; Kong, L.; et al. Ultrathin Light-Emitting Diodes with External Efficiency over 26% Based on Resurfaced Perovskite Nanocrystals. ACS Energy Lett. 2023, 8, 927–934. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, L.; Bai, J.; Wang, F.; Jiang, M. Heterointerface Engineering of Perovskite Defects and Energetics for Light-Emitting Diodes. Nano Res. 2023, 16, 5525–5532. [Google Scholar] [CrossRef]
- Kong, L.; Luo, Y.; Turyanska, L.; Zhang, T.; Zhang, Z.; Xing, G.; Yang, Y.; Zhang, C.; Yang, X. A Spacer Cation Assisted Nucleation and Growth Strategy Enables Efficient and High-Luminance Quasi-2D Perovskite LEDs. Adv. Funct. Mater. 2022, 33, 2209186. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, X.; Wang, F. Enabling Monodisperse Perovskite Phase with Buried Interface Modification Toward Efficient Light-Emitting Diodes. Nano Res. Energy 2023, 2, e9120069. [Google Scholar] [CrossRef]
- Pan, J.; Quan, L.N.; Zhao, Y.; Peng, W.; Murali, B.; Sarmah, S.P.; Yuan, M.; Sinatra, L.; Alyami, N.M.; Liu, J.; et al. Highly Efficient Perovskite-Quantum-Dot Light-Emitting Diodes by Surface Engineering. Adv. Mater. 2016, 28, 8718–8725. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, Y.K.; Yuan, F.; Johnston, A.; Liu, Y.; Ma, D.; Choi, M.J.; Chen, B.; Chekini, M.; Baek, S.W.; et al. Bipolar-Shell Resurfacing for Blue LEDs Based on Strongly Confined Perovskite Quantum Dots. Nat. Nanotechnol. 2020, 15, 668–674. [Google Scholar] [CrossRef]
- Koscher, B.A.; Swabeck, J.K.; Bronstein, N.D.; Alivisatos, A.P. Essentially Trap-Free CsPbBr3 Colloidal Nanocrystals by Postsynthetic Thiocyanate Surface Treatment. J. Am. Chem. Soc. 2017, 139, 6566–6569. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F.; Sun, W.; Ni, R.; Hu, S.; Liu, J.; Zhang, B.; Alsaed, A.; Hayat, T.; Tan, Z.a. Manipulating the Trade-off Between Quantum Yield and Electrical Conductivity for High-Brightness Quasi-2D Perovskite Light-Emitting Diodes. Adv. Funct. Mater. 2018, 28, 1804187. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Wang, T.; Song, J.; Chen, J.; Xue, J.; Dong, Y.; Cai, B.; Shan, Q.; Han, B.; et al. 50-Fold EQE Improvement up to 6.27% of Solution-Processed All-Inorganic Perovskite CsPbBr3 QLEDs via Surface Ligand Density Control. Adv. Mater. 2017, 29, 1603885. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, J.; Xu, L.; Li, J.; Zhang, F.; Han, B.; Shan, Q.; Zeng, H. Room-Temperature Triple-Ligand Surface Engineering Synergistically Boosts Ink Stability, Recombination Dynamics, and Charge Injection toward EQE-11.6% Perovskite QLEDs. Adv. Mater. 2018, 30, 1800764. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Hoshi, K.; Pu, Y.J.; Takeda, Y.; Hayashi, Y.; Ohisa, S.; Kawata, S.; Kido, J. High-Efficiency Perovskite Quantum-Dot Light-Emitting Devices by Effective Washing Process and Interfacial Energy Level Alignment. ACS Appl. Mater. Interfaces 2017, 9, 18054–18060. [Google Scholar] [CrossRef]
- Chen, H.; Fan, L.; Zhang, R.; Liu, W.; Zhang, Q.; Guo, R.; Zhuang, S.; Wang, L. Sodium Ion Modifying In Situ Fabricated CsPbBr3 Nanoparticles for Efficient Perovskite Light Emitting Diodes. Adv. Opt. Mater. 2019, 7, 1900747. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Wu, Q.; Cao, F.; Yang, D.; Shang, Y.; Ning, Z.; Zhang, W.; Zheng, W.; Yan, Y.; et al. Trifluoroacetate Induced Small-Grained CsPbBr3 Perovskite Films Result in Efficient and Stable Light-Emitting Devices. Nat. Commun. 2019, 10, 665–675. [Google Scholar] [CrossRef]
- Cui, J.; Liu, Y.; Deng, Y.; Lin, C.; Fang, Z.; Xiang, C.; Bai, P.; Du, K.; Zuo, X.; Wen, K.; et al. Efficient Light-Emitting Diodes Based on Oriented Perovskite Nanoplatelets. Sci. Adv. 2021, 7, 8458–8465. [Google Scholar] [CrossRef]
- Meggiolaro, D.; Motti, S.G.; Mosconi, E.; Barker, A.J.; Ball, J.; Andrea Riccardo Perini, C.; Deschler, F.; Petrozza, A.; De Angelis, F. Lodine Chemistry Determines the Defect Tolerance of Lead-Halide Perovskites. Energy Environ. Sci. 2018, 11, 702–713. [Google Scholar] [CrossRef]
- Pandey, M.; Rasmussen, F.A.; Kuhar, K.; Olsen, T.; Jacobsen, K.W.; Thygesen, K.S. Defect-Tolerant Monolayer Transition Metal Dichalcogenides. Nano Lett. 2016, 16, 2234–2240. [Google Scholar] [CrossRef]
- Chen, B.; Rudd, P.N.; Yang, S.; Yuan, Y.; Huang, J. Imperfections and Their Passivation in Halide Perovskite Solar Cells. Chem. Soc. Rev. 2019, 48, 3842–3867. [Google Scholar] [CrossRef]
- Ball, J.M.; Petrozza, A. Defects in Perovskite-Halides and Their Effects in Solar Cells. Nat. Energy 2016, 1, 16149–16162. [Google Scholar] [CrossRef]
- Liu, X.; Xu, W.; Bai, S.; Jin, Y.; Wang, J.; Friend, R.H.; Gao, F. Metal Halide Perovskites for Light-Emitting Diodes. Nat. Mater. 2021, 20, 10–21. [Google Scholar] [CrossRef] [PubMed]
- de Quilettes, D.W.; Koch, S.; Burke, S.; Paranji, R.K.; Shropshire, A.J.; Ziffer, M.E.; Ginger, D.S. Photoluminescence Lifetimes Exceeding 8 μs and Quantum Yields Exceeding 30% in Hybrid Perovskite Thin Films by Ligand Passivation. ACS Energy Lett. 2016, 1, 438–444. [Google Scholar] [CrossRef]
- Song, L.; Guo, X.; Hu, Y.; Lv, Y.; Lin, J.; Liu, Z.; Fan, Y.; Liu, X. Efficient Inorganic Perovskite Light-Emitting Diodes with Polyethylene Glycol Passivated Ultrathin CsPbBr3 Films. J. Phys. Chem. Lett. 2017, 8, 4148–4154. [Google Scholar] [CrossRef]
- Wu, C.; Zou, Y.; Wu, T.; Ban, M.; Pecunia, V.; Han, Y.; Liu, Q.; Song, T.; Duhm, S.; Sun, B. Improved Performance and Stability of All-Inorganic Perovskite Light-Emitting Diodes by Antisolvent Vapor Treatment. Adv. Funct. Mater. 2017, 27, 1700338. [Google Scholar] [CrossRef]
- Krieg, F.; Ochsenbein, S.T.; Yakunin, S.; Ten Brinck, S.; Aellen, P.; Suess, A.; Clerc, B.; Guggisberg, D.; Nazarenko, O.; Shynkarenko, Y.; et al. Colloidal CsPbX3 (X = Cl, Br, I) Nanocrystals 2.0: Zwitterionic Capping Ligands for Improved Durability and Stability. ACS Energy Lett. 2018, 3, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wan, Q.; Wang, B.; Zheng, W.; Liu, M.; Zhang, Q.; Kong, L.; Li, L. Surface Ligand Engineering toward Brightly Luminescent and Stable Cesium Lead Halide Perovskite Nanoplatelets for Efficient Blue-Light-Emitting Diodes. J. Phys. Chem. C. 2019, 123, 26161–26169. [Google Scholar] [CrossRef]
- Bae, W.K.; Park, Y.S.; Lim, J.; Lee, D.; Padilha, L.A.; McDaniel, H.; Robel, I.; Lee, C.; Pietryga, J.M.; Klimov, V.I. Controlling the Influence of Auger Recombination on the Performance of Quantum-Dot Light-Emitting Diodes. Nat. Commun. 2013, 4, 2661–2669. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Wang, G.; Ye, F.; Yan, H.; Zhang, M.; Dong, S.-C.; Lu, L.; Huang, P.; He, T.; et al. Lattice Strain Modulation Toward Efficient Blue Perovskite Light-Emitting Diodes. Sci. Adv. 2022, 8, 0138–0146. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Yang, Y.; Xue, Q.; Yip, H.L.; Cao, Y. Modulation of Recombination Zone Position for Quasi-Two-Dimensional Blue Perovskite Light-Emitting Diodes with Efficiency Exceeding 5%. Nat. Commun. 2019, 10, 1027–1037. [Google Scholar] [CrossRef]
- Ahmad, S.; Fu, P.; Yu, S.; Yang, Q.; Liu, X.; Wang, X.; Wang, X.; Guo, X.; Li, C. Dion-Jacobson Phase 2D Layered Perovskites for Solar Cells with Ultrahigh Stability. Joule 2019, 3, 794–806. [Google Scholar] [CrossRef]
- Yin, W.; Li, M.; Dong, W.; Luo, Z.; Li, Y.; Qian, J.; Zhang, J.; Zhang, W.; Zhang, Y.; Kershaw, S.V.; et al. Multidentate Ligand Polyethylenimine Enables Bright Color-Saturated Blue Light-Emitting Diodes Based on CsPbBr3 Nanoplatelets. ACS Energy Lett. 2021, 6, 477–484. [Google Scholar] [CrossRef]
- Bi, C.; Yao, Z.; Sun, X.; Wei, X.; Wang, J.; Tian, J. Perovskite Quantum Dots with Ultralow Trap Density by Acid Etching-Driven Ligand Exchange for High Luminance and Stable Pure-Blue Light-Emitting Diodes. Adv. Mater. 2021, 33, 2006722. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, X.; Gong, X.; Yin, J.; Zhou, D.; Sinatra, L.; Huang, R.; Liu, J.; Chen, J.; Dursun, I.; et al. Halogen Vacancies Enable Ligand-Assisted Self-Assembly of Perovskite Quantum Dots into Nanowires. Angew. Chem. Int. Ed. 2019, 58, 16077–16081. [Google Scholar] [CrossRef]
- Adhikari, G.C.; Vargas, P.A.; Zhu, H.; Grigoriev, A.; Zhu, P. Tetradic Phosphor White Light with Variable CCT and Superlative CRI through Organolead Halide Perovskite Nanocrystals. Nanoscale Adv. 2019, 1, 1791–1798. [Google Scholar] [CrossRef]
- Park, M.-H. 3D and 2D Metal Halide Perovskites for Blue Light-Emitting Diodes. Materials 2022, 15, 4571. [Google Scholar] [CrossRef]
- Adhikari, G.C.; Zhu, H.; Vargas, P.A.; Zhu, P. UV-Green Emission from Organolead Bromide Perovskite Nanocrystals. J. Phys. Chem. C 2018, 122, 15041–15046. [Google Scholar] [CrossRef]
- Chen, C.; Zeng, L.; Jiang, Z.; Xu, Z.; Chen, Y.; Wang, Z.; Chen, S.; Xu, B.; Mai, Y.; Guo, F. Vacuum-Assisted Preparation of High-Quality Quasi-2D Perovskite Thin Films for Large-Area Light-Emitting Diodes. Adv. Funct. Mater. 2022, 32, 2107644. [Google Scholar] [CrossRef]
- Ren, Z.; Li, L.; Yu, J.; Ma, R.; Xiao, X.; Chen, R.; Wang, K.; Sun, X.W.; Yin, W.-J.; Choy, W.C.H. Simultaneous Low-Order Phase Suppression and Defect Passivation for Efficient and Stable Blue Light-Emitting Diodes. ACS Energy Lett. 2020, 5, 2569–2579. [Google Scholar] [CrossRef]
- Ren, Z.; Xiao, X.; Ma, R.; Lin, H.; Wang, K.; Sun, X.W.; Choy, W.C.H. Hole Transport Bilayer Structure for Quasi-2D Perovskite Based Blue Light-Emitting Diodes with High Brightness and Good Spectral Stability. Adv. Funct. Mater. 2019, 29, 1905339. [Google Scholar] [CrossRef]
- Ren, Z.; Yu, J.; Qin, Z.; Wang, J.; Sun, J.; Chan, C.C.S.; Ding, S.; Wang, K.; Chen, R.; Wong, K.S.; et al. High-Performance Blue Perovskite Light-Emitting Diodes Enabled by Efficient Energy Transfer between Coupled Quasi-2D Perovskite Layers. Adv. Mater. 2021, 33, 2005570. [Google Scholar] [CrossRef]
- Wang, Y.K.; Ma, D.; Yuan, F.; Singh, K.; Pina, J.M.; Johnston, A.; Dong, Y.; Zhou, C.; Chen, B.; Sun, B.; et al. Chelating-Agent-Assisted Control of CsPbBr3 Quantum Well Growth Enables Stable Blue Perovskite Emitters. Nat. Commun. 2020, 11, 3674–3681. [Google Scholar] [CrossRef]
- Bekenstein, Y.; Koscher, B.A.; Eaton, S.W.; Yang, P.; Alivisatos, A.P. Highly Luminescent Colloidal Nanoplates of Perovskite Cesium Lead Halide and Their Oriented Assemblies. J. Am. Chem. Soc. 2015, 137, 16008–16011. [Google Scholar] [CrossRef]
- Song, J.; Xu, L.; Li, J.; Xue, J.; Dong, Y.; Li, X.; Zeng, H. Monolayer and Few-Layer All-Inorganic Perovskites as a New Family of Two-Dimensional Semiconductors for Printable Optoelectronic Devices. Adv. Mater. 2016, 28, 4861–4869. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q.A.; Motti, S.G.; Srimath Kandada, A.R.; Mosconi, E.; D’Innocenzo, V.; Bertoni, G.; Marras, S.; Kamino, B.A.; Miranda, L.; De Angelis, F.; et al. Solution Synthesis Approach to Colloidal Cesium Lead Halide Perovskite Nanoplatelets with Monolayer-Level Thickness Control. J. Am. Chem. Soc. 2016, 138, 1010–1016. [Google Scholar] [CrossRef]
- Yang, D.; Zou, Y.; Li, P.; Liu, Q.; Wu, L.; Hu, H.; Xu, Y.; Sun, B.; Zhang, Q.; Lee, S.-T. Large-Scale Synthesis of Ultrathin Cesium Lead Bromide Perovskite Nanoplates with Precisely Tunable Dimensions and Their Application in Blue Light-Emitting Diodes. Nano Energy 2018, 47, 235–242. [Google Scholar] [CrossRef]
- Liu, H.; Worku, M.; Mondal, A.; Shonde, T.B.; Chaaban, M.; Ben-Akacha, A.; Lee, S.; Gonzalez, F.; Olasupo, O.; Lin, X.; et al. Efficient and Stable Blue Light Emitting Diodes Based on CsPbBr3 Nanoplatelets with Surface Passivation by Multifunctional Organic Sulfate. Adv. Energy Mater. 2022, 12, 2201605. [Google Scholar] [CrossRef]
- Shen, W.; Yu, Y.; Zhang, W.; Chen, Y.; Zhang, J.; Yang, L.; Feng, J.; Cheng, G.; Liu, L.; Chen, S. Efficient Pure Blue Light-Emitting Diodes Based on CsPbBr3 Quantum-Confined Nanoplates. ACS Appl. Mater. Interfaces 2022, 14, 5682–5691. [Google Scholar] [CrossRef] [PubMed]
- Hoye, R.L.Z.; Lai, M.L.; Anaya, M.; Tong, Y.; Galkowski, K.; Doherty, T.; Li, W.; Huq, T.N.; Mackowski, S.; Polavarapu, L.; et al. Identifying and Reducing Interfacial Losses to Enhance Color-Pure Electroluminescence in Blue-Emitting Perovskite Nanoplatelet Light-Emitting Diodes. ACS Energy Lett. 2019, 4, 1181–1188. [Google Scholar] [CrossRef]
- Wang, H.; Ye, F.; Sun, J.; Wang, Z.; Zhang, C.; Qian, J.; Zhang, X.; Choy, W.C.H.; Sun, X.W.; Wang, K.; et al. Efficient CsPbBr3 Nanoplatelet-Based Blue Light-Emitting Diodes Enabled by Engineered Surface Ligands. ACS Energy Lett. 2022, 7, 1137–1145. [Google Scholar] [CrossRef]
- Bi, C.; Yao, Z.; Hu, J.; Wang, X.; Zhang, M.; Tian, S.; Liu, A.; Lu, Y.; de Leeuw, N.H.; Sui, M.; et al. Suppressing Auger Recombination of Perovskite Quantum Dots for Efficient Pure-Blue-Light-Emitting Diodes. ACS Energy Lett. 2022, 8, 731–739. [Google Scholar] [CrossRef]
- Yao, Z.; Bi, C.; Liu, A.; Zhang, M.; Tian, J. High Brightness and Stability Pure-Blue Perovskite Light-Emitting Diodes Based on a Novel Structural Quantum-Dot Film. Nano Energy 2022, 95, 106982. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, C.; Xu, J.; Li, S.; Cui, M.; Fu, X.; Liu, Y.; Liu, Y.; Wan, H.; Wei, K.; et al. Synthesis-on-Substrate of Quantum Dot Solids. Nature 2022, 612, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wei, C.; Li, X.; Li, Y.; Qiu, S.; Shen, W.; Cai, B.; Sun, Z.; Yang, D.; Deng, Z.; et al. In Situ Passivation of [PbBr6]4– Octahedra toward Blue Luminescent CsPbBr3 Nanoplatelets with Near 100% Absolute Quantum Yield. ACS Energy Lett. 2018, 3, 2030–2037. [Google Scholar] [CrossRef]
- Klein-Kedem, N.; Cahen, D.; Hodes, G. Effects of Light and Electron Beam Irradiation on Halide Perovskites and Their Solar Cells. Acc. Chem. Res. 2016, 49, 347–354. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, Y.; Zhou, X.; Fu, R.; Li, Q.; Zhao, Y.; Liu, K.; Yu, D.; Zhao, Q. Light-Independent Ionic Transport in Inorganic Perovskite and Ultrastable Cs-Based Perovskite Solar Cells. J. Phys. Chem. Lett. 2017, 8, 4122–4128. [Google Scholar] [CrossRef]
- Akbulatov, A.F.; Luchkin, S.Y.; Frolova, L.A.; Dremova, N.N.; Gerasimov, K.L.; Zhidkov, I.S.; Anokhin, D.V.; Kurmaev, E.Z.; Stevenson, K.J.; Troshin, P.A. Probing the Intrinsic Thermal and Photochemical Stability of Hybrid and Inorganic Lead Halide Perovskites. J. Phys. Chem. Lett. 2017, 8, 1211–1218. [Google Scholar] [CrossRef]
- Chen, J.; Liu, D.; Al-Marri, M.J.; Nuuttila, L.; Lehtivuori, H.; Zheng, K. Photo-Stability of CsPbBr3 Perovskite Quantum Dots for Optoelectronic Application. Sci. China Mater. 2016, 59, 719–727. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, G.; Jiang, L.; Cai, Y.; Gao, Y.; Yang, W.; He, X.; Zeng, Q.; Xing, G.; Jia, Y.; et al. Water, a Green Solvent for Fabrication of High-Quality CsPbBr3 Films for Efficient Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 5925–5931. [Google Scholar] [CrossRef]
- Dyrvik, E.G.; Warby, J.H.; McCarthy, M.M.; Ramadan, A.J.; Zaininger, K.A.; Lauritzen, A.E.; Mahesh, S.; Taylor, R.A.; Snaith, H.J. Reducing Nonradiative Losses in Perovskite LEDs through Atomic Layer Deposition of Al2O3 on the Hole-Injection Contact. ACS Nano 2023, 17, 3289–3300. [Google Scholar] [CrossRef]
- Kim, J.S.; Heo, J.M.; Park, G.S.; Woo, S.J.; Cho, C.; Yun, H.J.; Kim, D.H.; Park, J.; Lee, S.C.; Park, S.H.; et al. Ultra-Bright, Efficient and Stable Perovskite Light-Emitting Diodes. Nature 2022, 611, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Huang, Z.; Yao, H.; Liu, Y.; Zhang, Y.; Li, Z.; Zhou, H.; Xiao, P.; Chen, T.; Sun, H.; et al. Highly Bright and Stable Single-Crystal Perovskite Light-Emitting Diodes. Nat. Photonics 2023, 17, 401–407. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Li, P.; Liu, J.; Liang, L.; Wang, L.; Ding, Y.; Han, X.; Ji, J.; Chen, S.; et al. The Universal Growth of Ultrathin Perovskite Single Crystals. Adv. Mater. 2022, 34, 2108396. [Google Scholar] [CrossRef] [PubMed]




| Year | Emitting Layer | EQE (%) | Brightness (cd m−2) | EL Peak (nm) | Stability (min) | Ref. |
|---|---|---|---|---|---|---|
| 2018 | HBr-treated CsPbBr3 NPL | 0.124 | 62 | 463 | - | [94] |
| 2019 | poly(triarylamine) modified CsPbBr3 NPL | 0.3 | - | 464 | - | [89] |
| 2019 | DDAB-treated CsPbBr3 NPL | 1.42 | 41.8 | 469 | 0.7 | [67] |
| 2021 | PEI-modified CsPbBr3 NPL | 0.8 | 631 | 465 | - | [72] |
| 2022 | SA-modified CsPbBr3 NPL | 3.18 | 81.8 | 460 | 6.2 | [88] |
| 2022 | NH4Br- and PEABr-modified CsPbBr3 NPL | 2 | 74 | 463 | - | [90] |
| 2022 | EDBeSO4-modified CsPbBr3 NPL | 1.77 | 691 | 462 | 20 | [87] |
| 2019 | PA2(CsPb Br3)n−1PbBr4 | 1.45 | 5735 | 487 | 220 at 150 cd m−2 | [80] |
| 2020 | PEAxPA2−x(CsPbBr3)n−1PbBr4 | 7.51 | 1765 | 488 | 66 | [79] |
| 2020 | GABA-treated PEA2(CsPbBr3)n−1PbBr4 | 6.3 | 200 | 478 | 2.5 at 200 cd m−2 | [82] |
| 2020 | ABA2PbBr4-modified PEAxPA2−x(CsPbBr3)n−1PbBr4 | 11.1 | 513 | 486 | 81.3 | [81] |
| 2020 | Bipolar-shell-protected 4 nm CsPbBr3 QDs | 12.3 | ~450 | 479 | 20 at 90 cd m−2 | [48] |
| 2021 | DDDAM- and PEA-treated 4 nm CsPbBr3 QDs | 4.7 | 3850 | 470 | 720 | [73] |
| 2022 | ZnBr2-treated 4 nm CsPbBr3 QDs | 10.3 | 12060 | 469 | 1500 at 115 cd m−2 | [91] |
| 2022 | Hydrobromide-treated CsPbBr3 QDs | 6.6 | 280.8 | 480 | 1.83 at 80 cd m−2 | [28] |
| 2022 | Br-MBA+-treated CsPbBr3 quantum dots | 17.9% | ~2500 | 480 | 120 at 100 cd m−2 | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Wang, F. Toward High-Performances of Halide Light-Emitting Diodes: The Importance of Ligands Engineering. Inorganics 2023, 11, 230. https://doi.org/10.3390/inorganics11060230
Ma L, Wang F. Toward High-Performances of Halide Light-Emitting Diodes: The Importance of Ligands Engineering. Inorganics. 2023; 11(6):230. https://doi.org/10.3390/inorganics11060230
Chicago/Turabian StyleMa, Le, and Feijiu Wang. 2023. "Toward High-Performances of Halide Light-Emitting Diodes: The Importance of Ligands Engineering" Inorganics 11, no. 6: 230. https://doi.org/10.3390/inorganics11060230
APA StyleMa, L., & Wang, F. (2023). Toward High-Performances of Halide Light-Emitting Diodes: The Importance of Ligands Engineering. Inorganics, 11(6), 230. https://doi.org/10.3390/inorganics11060230