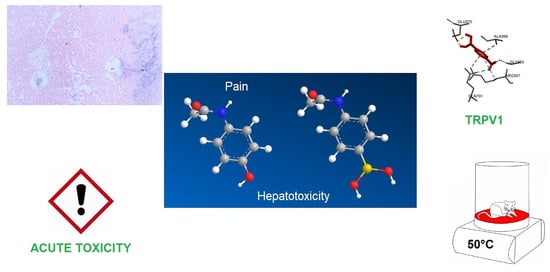
A Boron-Containing Analogue of Acetaminophen Induces Analgesic Effect in Hot Plate Test and Limited Hepatotoxicity
Abstract
:1. Introduction
2. Results
2.1. Docking Assays and In Silico Kinetic Evaluation
2.2. Determination of Intraperitoneal LD50 of 4APB and In Silico Toxicity Prediction
2.3. Hot Plate and Motor Activity
2.4. Hepatotoxicity and Liver Regeneration
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. Retrieval of Tested Structures
4.3. Docking Procedure
4.4. In Silico and In Vivo Toxicity Evaluation
4.5. Hot Plate Test
4.6. Open Field Test
4.7. Partial Hepatectomy Model
4.8. Liver Histology
4.9. Determination of Enzymes and Metabolites in Serum
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A. The revised IASP definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef] [Green Version]
- Moriya, S.; Yamashita, A.; Nishi, R.; Ikoma, Y.; Yamanaka, A.; Kuwaki, T. Acute nociceptive stimuli rapidly induce the activity of serotonin and noradrenalin neurons in the brain stem of awake mice. IBRO Rep. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 2013, 504, 107–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jara-Oseguera, A.; Simon, S.A.; Rosenbaum, T. TRPV1: On the road to pain relief. Curr. Mol. Pharmacol. 2008, 1, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.; Sheth, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. TRPV1: A potential drug target for treating various diseases. Cells 2014, 3, 517–545. [Google Scholar] [CrossRef] [Green Version]
- Gambino, G.; Rizzo, V.; Giglia, G.; Ferraro, G.; Sardo, P. Cannabinoids, TRPV and nitric oxide: The three ring circus of neuronal excitability. Brain Struct. Funct. 2020, 225, 1–15. [Google Scholar] [CrossRef]
- Maione, S.; Starowicz, K.; Cristino, L.; Guida, F.; Palazzo, E.; Luongo, L.; Rossi, F.; Marabese, I.; de Novellis, V.; Di Marzo, V. Functional interaction between TRPV1 and μ-opioid receptors in the descending antinociceptive pathway activates glutamate transmission and induces analgesia. J. Neurophysiol. 2009, 101, 2411–2422. [Google Scholar] [CrossRef] [Green Version]
- Morgan, M.M.; Whittier, K.L.; Hegarty, D.M.; Aicher, S.A. Periaqueductal gray neurons project to spinally projecting GABAergic neurons in the rostral ventromedial medulla. Pain 2008, 140, 376–386. [Google Scholar] [CrossRef] [Green Version]
- Yanarates, O.; Dogrul, A.; Yildirim, V.; Sahin, A.; Sizlan, A.; Seyrek, M.; Akgül, Ö.; Kozak, O.; Kurt, E.; Aypar, U. Spinal 5-HT7 receptors play an important role in the antinociceptive and antihyperalgesic effects of tramadol and its metabolite, O-Desmethyltramadol, via activation of descending serotonergic pathways. J. Am. Soc. Anesthesiol. 2010, 112, 696–710. [Google Scholar] [CrossRef] [Green Version]
- Holloway, B.B.; Stornetta, R.L.; Bochorishvili, G.; Erisir, A.; Viar, K.E.; Guyenet, P.G. Monosynaptic glutamatergic activation of locus coeruleus and other lower brainstem noradrenergic neurons by the C1 cells in mice. J. Neurosci. 2013, 33, 18792–18805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenchat, A.; Nadal, X.; Romero, L.; Ovalle, S.; Muro, A.; Sánchez-Arroyos, R.; Portillo-Salido, E.; Pujol, M.; Montero, A.; Codony, X. Pharmacological activation of 5-HT7 receptors reduces nerve injury-induced mechanical and thermal hypersensitivity. Pain 2010, 149, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Llorca-Torralba, M.; Borges, G.; Neto, F.; Mico, J.A.; Berrocoso, E. Noradrenergic Locus Coeruleus pathways in pain modulation. Neuroscience 2016, 338, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Hodgman, M.J.; Garrard, A.R. A review of acetaminophen poisoning. Crit. Care Clin. 2012, 28, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Jaeschke, H. Acetaminophen hepatotoxicity: A mitochondrial perspective. Adv. Pharmacol. 2019, 85, 195–219. [Google Scholar]
- Marin, T.M.; de Carvalho Indolfo, N.; Rocco, S.A.; Basei, F.L.; de Carvalho, M.; de Almeida Gonçalves, K.; Pagani, E. Acetaminophen absorption and metabolism in an intestine/liver microphysiological system. Chem. Biol. Interact. 2019, 299, 59–76. [Google Scholar] [CrossRef]
- McGill, M.R.; Jaeschke, H. Metabolism and disposition of acetaminophen: Recent advances in relation to hepatotoxicity and diagnosis. Pharm. Res. 2013, 30, 2174–2187. [Google Scholar] [CrossRef] [Green Version]
- Graham, G.G.; Davies, M.J.; Day, R.O.; Mohamudally, A.; Scott, K.F. The modern pharmacology of paracetamol: Therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology 2013, 21, 201–232. [Google Scholar] [CrossRef]
- Barbier-Torres, L.; Iruzubieta, P.; Fernández-Ramos, D.; Delgado, T.C.; Taibo, D.; Guitiérrez-de-Juan, V.; Varela-Rey, M.; Azkargorta, M.; Navasa, N.; Fernández-Tussy, P. The mitochondrial negative regulator MCJ is a therapeutic target for acetaminophen-induced liver injury. Nat. Commun. 2017, 8, 2068. [Google Scholar] [CrossRef]
- Stueber, T.; Meyer, S.; Jangra, A.; Hage, A.; Eberhardt, M.; Leffler, A. Activation of the capsaicin-receptor TRPV1 by the acetaminophen metabolite N-arachidonoylaminophenol results in cytotoxicity. Life Sci. 2018, 194, 67–74. [Google Scholar] [CrossRef]
- Caballero, F.J.; Soler-Torronteras, R.; Lara-Chica, M.; García, V.; Fiebich, B.L.; Muñoz, E.; Calzado, M.A. AM404 inhibits NFAT and NF-κB signaling pathways and impairs migration and invasiveness of neuroblastoma cells. Eur. J. Pharmacol. 2015, 746, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.V.; Long, J.H.; Shah, S.; Rahman, J.; Perrett, D.; Ayoub, S.S.; Mehta, V. First evidence of the conversion of paracetamol to AM404 in human cerebrospinal fluid. J. Pain Res. 2017, 10, 2703–2709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Högestätt, E.D.; Jönsson, B.A.; Ermund, A.; Andersson, D.A.; Björk, H.; Alexander, J.P.; Cravatt, B.F.; Basbaum, A.I.; Zygmunt, P.M. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J. Biol. Chem. 2005, 280, 31405–31412. [Google Scholar] [CrossRef] [Green Version]
- Saliba, S.W.; Bonifacino, T.; Serchov, T.; Bonanno, G.; de Oliveira, A.C.P.; Fiebich, B.L. Neuroprotective effect of AM404 against NMDA-induced hippocampal excitotoxicity. Front. Cell. Neurosci. 2019, 13, 566. [Google Scholar] [CrossRef] [Green Version]
- Abu Rmilah, A.; Zhou, W.; Nelson, E.; Lin, L.; Amiot, B.; Nyberg, S.L. Understanding the marvels behind liver regeneration. Wiley Interdiscip. Rev. Dev. Biol. 2019, 8, e340. [Google Scholar] [CrossRef]
- Behari, J. The Wnt/β-catenin signaling pathway in liver biology and disease. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Moroishi, T.; Guan, K.-L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.W.; Kim, Y.C.; Yu, B.O.; Moroishi, T.; Mo, J.-S.; Plouffe, S.W.; Meng, Z.; Lin, K.C.; Yu, F.-X.; Alexander, C.M. Alternative Wnt signaling activates YAP/TAZ. Cell 2015, 162, 780–794. [Google Scholar] [CrossRef] [Green Version]
- Elpek, G.Ö. Angiogenesis and liver fibrosis. World J. Hepatol. 2015, 7, 377. [Google Scholar] [CrossRef]
- Ebrahem, Q.; Chaurasia, S.S.; Vasanji, A.; Qi, J.H.; Klenotic, P.A.; Cutler, A.; Asosingh, K.; Erzurum, S.; Anand-Apte, B. Cross-talk between vascular endothelial growth factor and matrix metalloproteinases in the induction of neovascularization in vivo. Am. J. Pathol. 2010, 176, 496–503. [Google Scholar] [CrossRef] [Green Version]
- Norden, P.R.; Kim, D.J.; Barry, D.M.; Cleaver, O.B.; Davis, G.E. Cdc42 and k-Ras control endothelial tubulogenesis through apical membrane and cytoskeletal polarization: Novel stimulatory roles for GTPase effectors, the small GTPases, Rac2 and Rap1b, and inhibitory influence of Arhgap31 and Rasa1. PLoS ONE 2016, 11, e0147758. [Google Scholar] [CrossRef] [Green Version]
- Hakanpaa, L.; Sipila, T.; Leppanen, V.-M.; Gautam, P.; Nurmi, H.; Jacquemet, G.; Eklund, L.; Ivaska, J.; Alitalo, K.; Saharinen, P. Endothelial destabilization by angiopoietin-2 via integrin β1 activation. Nat. Commun. 2015, 6, 5962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strazzabosco, M.; Fabris, L. Development of the bile ducts: Essentials for the clinical hepatologist. J. Hepatol. 2012, 56, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Ursúa, M.A.; Das, B.C.; Trujillo-Ferrara, J.G. Boron-containing compounds: Chemico-biological properties and expanding medicinal potential in prevention, diagnosis and therapy. Expert Opin. Ther. Pat. 2014, 24, 485–500. [Google Scholar] [CrossRef]
- Rosalez, M.N.; Estevez-Fregoso, E.; Alatorre, A.; Abad-García, A.; Soriano-Ursúa, M.A. 2-Aminoethyldiphenyl Borinate: A Multitarget Compound with Potential as a Drug Precursor. Curr. Mol. Pharmacol. 2020, 13, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef]
- Kane, A.E.; Mitchell, S.J.; Mach, J.; Huizer-Pajkos, A.; McKenzie, C.; Jones, B.; Cogger, V.; Le Couteur, D.G.; de Cabo, R.; Hilmer, S.N. Acetaminophen hepatotoxicity in mice: Effect of age, frailty and exposure type. Exp. Gerontol. 2016, 73, 95–106. [Google Scholar] [CrossRef]
- Lu, H.-H.; Thomas, J.D.; Tukker, J.J.; Fleisher, D. Intestinal water and solute absorption studies: Comparison of in situ perfusion with chronic isolated loops in rats. Pharm. Res. 1992, 9, 894–900. [Google Scholar] [CrossRef]
- Ocampo-Néstor, A.L.; Trujillo-Ferrara, J.G.; Abad-García, A.; Reyes-López, C.; Geninatti-Crich, S.; Soriano-Ursua, M.A. Boron’s journey: Advances in the study and application of pharmacokinetics. Expert Opin. Ther. Pat. 2017, 27, 203–215. [Google Scholar] [CrossRef]
- Zhao, L.; Pickering, G. Paracetamol metabolism and related genetic differences. Drug Metab. Rev. 2011, 43, 41–52. [Google Scholar] [CrossRef]
- Tjølsen, A.; Rosland, J.H.; Berge, O.-G.; Hole, K. The increasing-temperature hot-plate test: An improved test of nociception in mice and rats. J. Pharmacol. Methods 1991, 25, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Seibenhener, M.L.; Wooten, M.C. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. JoVE 2015, 52434. [Google Scholar] [CrossRef] [Green Version]
- Maahs, D.M.; DeSalvo, D.; Pyle, L.; Ly, T.; Messer, L.; Clinton, P.; Westfall, E.; Wadwa, R.P.; Buckingham, B. Effect of acetaminophen on CGM glucose in an outpatient setting. Diabetes Care 2015, 38, e158–e159. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Veettil, S.; Dyer, R.; Peyser, T.; Basu, R. Direct evidence of acetaminophen interference with subcutaneous glucose sensing in humans: A pilot study. Diabetes Technol. Ther. 2016, 18, S2–S43. [Google Scholar] [CrossRef] [Green Version]
- Donoiu, I.; Militaru, C.; Obleagă, O.; Hunter, J.M.; Neamţu, J.; Biţă, A.; Scorei, I.R.; Rogoveanu, O.C. Effects of boron-containing compounds on cardiovascular disease risk factors—A review. J. Trace Elem. Med. Biol. 2018, 50, 47–56. [Google Scholar] [CrossRef]
- López-Cabrera, Y.; Castillo-García, E.L.; Altamirano-Espino, J.A.; Pérez-Capistran, T.; Farfán-García, E.D.; Trujillo-Ferrara, J.G.; Soriano-Ursúa, M.A. Profile of three boron-containing compounds on the body weight, metabolism and inflammatory markers of diabetic rats. J. Trace Elem. Med. Biol. 2018, 50, 424–429. [Google Scholar] [CrossRef]
- Keshet, R.; Szlosarek, P.; Carracedo, A.; Erez, A. Rewiring urea cycle metabolism in cancer to support anabolism. Nat. Rev. Cancer 2018, 18, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Kazak, L.; Cohen, P. Creatine metabolism: Energy homeostasis, immunity and cancer biology. Nat. Rev. Endocrinol. 2020, 16, 421–436. [Google Scholar] [CrossRef]
- Syal, K.; Banerjee, D.; Srinivasan, A. Creatinine estimation and interference. Indian J. Clin. Biochem. 2013, 28, 210–211. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
- Estevez-Fregoso, E.; Kilic, A.; Rodríguez-Vera, D.; Nicanor-Juárez, L.E.; Romero-Rizo, C.E.M.; Farfán-García, E.D.; Soriano-Ursúa, M.A. Effects of Boron-Containing Compounds on Liposoluble Hormone Functions. Inorganics 2023, 11, 84. [Google Scholar] [CrossRef]
- Röhrl, C.; Stangl, H. Cholesterol metabolism—Physiological regulation and pathophysiological deregulation by the endoplasmic reticulum. Wien. Med. Wochenschr. 2018, 168, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özsoy, M.B.; Pabuçcuoğlu, A. The effect of acetaminophen on oxidative modification of low-density lipoproteins in hypercholesterolemic rabbits. J. Clin. Biochem. Nutr. 2007, 41, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, T.; Greenspan, P. Effect of acetaminophen on the myeloperoxidase–hydrogen peroxide–nitrite mediated oxidation of LDL. Biochim. Biophys. Acta (BBA)-Molecular Cell Biol. Lipids 2002, 1581, 57–63. [Google Scholar] [CrossRef]
- Herrington, W.; Illingworth, N.; Staplin, N.; Kumar, A.; Storey, B.; Hrusecka, R.; Judge, P.; Mahmood, M.; Parish, S.; Landray, M. Effect of processing delay and storage conditions on urine albumin-to-creatinine ratio. Clin. J. Am. Soc. Nephrol. 2016, 11, 1794–1801. [Google Scholar] [CrossRef] [Green Version]
- Levitt, D.G.; Levitt, M.D. Human serum albumin homeostasis: A new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int. J. Gen. Med. 2016, 9, 229–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin—More than just a serum protein. Front. Physiol. 2014, 5, 299. [Google Scholar] [CrossRef] [Green Version]
- Ekam, V.S.; Udosen, E.O. Total protein, albumin and globulin levels following the administration of activity directed fractions of Vernonia amygdalina during acetaminophen induced hepatotoxicity in Wistar albino rats. Glob. J. Pure Appl. Sci. 2012, 18, 25–29. [Google Scholar]
- Prinville, V.; Ohlund, L.; Sleno, L. Targeted analysis of 46 bile acids to study the effect of acetaminophen in rat by LC-MS/MS. Metabolites 2020, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Al-Doaiss, A.A. Hepatotoxicity-Induced by the therapeutic dose of acetaminophen and the ameliorative effect of oral co-administration of selenium/Tribulus terrestris extract in rats. Int. J. Morphol. 2020, 38, 1444–1454. [Google Scholar] [CrossRef]
- Manautou, J.E.; De Waart, D.R.; Kunne, C.; Zelcer, N.; Goedken, M.; Borst, P.; Elferink, R.O. Altered disposition of acetaminophen in mice with a disruption of the Mrp3 gene. Hepatology 2005, 42, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tracy, T.S.; Remmel, R.P. Bilirubin glucuronidation revisited: Proper assay conditions to estimate enzyme kinetics with recombinant UGT1A1. Drug Metab. Dispos. 2010, 38, 1907–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.; Chen, H.; He, H.; Ma, C. Assays for alkaline phosphatase activity: Progress and prospects. TrAC Trends Anal. Chem. 2019, 113, 32–43. [Google Scholar] [CrossRef]
- Wicaksono, S.A.; Mardin, A.M.F.; Utami, S.B. The Effect of Paracetamol and Codeine Analgesic Combination on Serum Alanine Aminotransferase and Aspartate Aminotransferase Levels in Male Wistar Rats. Open Access Maced. J. Med. Sci. 2022, 10, 2267–2272. [Google Scholar] [CrossRef]
- Çelik, M.; Aydin, P. Investigation of the effect of 4-hydroxyphenylboronic acid on acetaminophen-induced liver cell injury in HEPG2 cell line. J. Boron 2022, 7, 507–513. [Google Scholar] [CrossRef]
- Nevzorova, Y.A.; Tolba, R.; Trautwein, C.; Liedtke, C. Partial hepatectomy in mice. Lab. Anim. 2015, 49, 81–88. [Google Scholar] [CrossRef]
- Marongiu, F.; Marongiu, M.; Contini, A.; Serra, M.; Cadoni, E.; Murgia, R.; Laconi, E. Hyperplasia vs hypertrophy in tissue regeneration after extensive liver resection. World J. Gastroenterol. 2017, 23, 1764. [Google Scholar] [CrossRef] [Green Version]
- Andersen, K.J.; Knudsen, A.R.; Kannerup, A.-S.; Sasanuma, H.; Nyengaard, J.R.; Hamilton-Dutoit, S.; Erlandsen, E.J.; Jørgensen, B.; Mortensen, F.V. The natural history of liver regeneration in rats: Description of an animal model for liver regeneration studies. Int. J. Surg. 2013, 11, 903–908. [Google Scholar] [CrossRef] [Green Version]
- Kaware, M. Changes in liver and body weight of mice exposed to toxicant. Int. J. Sci. Eng 2013, 3, 92–95. [Google Scholar]
- Böhm, F.; Köhler, U.A.; Speicher, T.; Werner, S. Regulation of liver regeneration by growth factors and cytokines. EMBO Mol. Med. 2010, 2, 294–305. [Google Scholar] [CrossRef]
- Li, J.-X.; Fei, X.-M.; Lu, H.; Hu, H.-J.; Li, J.-Y. Effect of proteasome inhibitor on migration ability and hepatocyte growth factor expression of bone marrow mesenchymal stem cells in multiple myeloma patients. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2011, 19, 1204–1208. [Google Scholar] [PubMed]
- Tee, L.B.G.; Davies, D.S.; Seddon, C.E.; Boobis, A.R. Species differences in the hepatotoxicity of paracetamol are due to differences in the rate of conversion to its cytotoxic metabolite. Biochem. Pharmacol. 1987, 36, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A.L.; Leach, M.C.; Flecknell, P.A. The analgesic effects of oral paracetamol in two strains of mice undergoing vasectomy. Lab. Anim. 2009, 43, 357–361. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Cao, E.; Julius, D.; Cheng, Y. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 2016, 534, 347–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soriano-Ursúa, M.A.; Bello, M.; Hernández-Martínez, C.F.; Santillán-Torres, I.; Guerrero-Ramírez, R.; Correa-Basurto, J.; Arias-Montaño, J.A.; Trujillo-Ferrara, J.G. Cell-based assays and molecular dynamics analysis of a boron-containing agonist with different profiles of binding to human and guinea pig beta2 adrenoceptors. Eur. Biophys. J. 2019, 48, 83–97. [Google Scholar] [CrossRef]
- Farfán-García, E.D.; Rosales-Hernández, M.C.; Castillo-García, E.L.; Abad-García, A.; Ruiz-Maciel, O.; Velasco-Silveyra, L.M.; González-Muñiz, A.Y.; Andrade-Jorge, E.; Soriano-Ursúa, M.A. Identification and evaluation of boronic compounds ameliorating cognitive deficit in orchiectomized rats. J. Trace Elem. Med. Biol. 2022, 72, 126979. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Popov, I.A.; Boldyrev, A.I. Classical and Multicenter Bonding in Boron: Two Faces of Boron. In Boron. Challenges and Advances in Computational Chemistry and Physics; Hnyk, D., McKee, M., Eds.; Springer: Cham, Switzerland, 2015; Volume 20, pp. 1–16. [Google Scholar] [CrossRef]
- Chinedu, E.; Arome, D.; Solomon Ameh, F. A new method for determining acute toxicity in animal models. Toxicol. Int. 2013, 20, 224–226. [Google Scholar] [CrossRef] [Green Version]
- Higgins, G. Experimental pathology of the liver. Restoration of the liver of the white rat following partial surgical removal. AMA Arch Pathol 1931, 12, 186–202. [Google Scholar]
- Madrigal-Santillán, E.; Bautista, M.; Gayosso-De-Lucio, J.A.; Reyes-Rosales, Y.; Posadas-Mondragón, A.; Morales-González, Á.; Soriano-Ursúa, M.A.; García-Machorro, J.; Madrigal-Bujaidar, E.; Álvarez-González, I.; et al. Hepatoprotective effect of Geranium schiedeanum against ethanol toxicity during liver regeneration. World J. Gastroenterol. 2015, 21, 7718. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosalez, M.N.; Farfán-García, E.D.; Badillo-Romero, J.; Córdova-Chávez, R.I.; Trujillo-Ferrara, J.G.; Morales-González, J.A.; Soriano-Ursúa, M.A.; Martínez-Archundia, M. A Boron-Containing Analogue of Acetaminophen Induces Analgesic Effect in Hot Plate Test and Limited Hepatotoxicity. Inorganics 2023, 11, 261. https://doi.org/10.3390/inorganics11060261
Rosalez MN, Farfán-García ED, Badillo-Romero J, Córdova-Chávez RI, Trujillo-Ferrara JG, Morales-González JA, Soriano-Ursúa MA, Martínez-Archundia M. A Boron-Containing Analogue of Acetaminophen Induces Analgesic Effect in Hot Plate Test and Limited Hepatotoxicity. Inorganics. 2023; 11(6):261. https://doi.org/10.3390/inorganics11060261
Chicago/Turabian StyleRosalez, Melvin Nadir, Eunice D. Farfán-García, Jesús Badillo-Romero, Ricardo Iván Córdova-Chávez, José G. Trujillo-Ferrara, José A. Morales-González, Marvin A. Soriano-Ursúa, and Marlet Martínez-Archundia. 2023. "A Boron-Containing Analogue of Acetaminophen Induces Analgesic Effect in Hot Plate Test and Limited Hepatotoxicity" Inorganics 11, no. 6: 261. https://doi.org/10.3390/inorganics11060261
APA StyleRosalez, M. N., Farfán-García, E. D., Badillo-Romero, J., Córdova-Chávez, R. I., Trujillo-Ferrara, J. G., Morales-González, J. A., Soriano-Ursúa, M. A., & Martínez-Archundia, M. (2023). A Boron-Containing Analogue of Acetaminophen Induces Analgesic Effect in Hot Plate Test and Limited Hepatotoxicity. Inorganics, 11(6), 261. https://doi.org/10.3390/inorganics11060261