Enhanced Lithium Storage Performance in Si/MXene Porous Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Ti3C2Tx MXene
2.2. Fabrication of NH2-Si/MXene Composite
2.3. Material Characterization
2.4. Electrochemical Measurement
3. Results and Discussion
3.1. Material Characterization
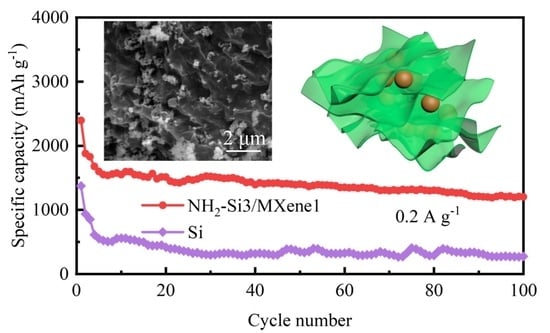
3.2. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Szczech, J.R.; Jin, S. Nanostructured silicon for high capacity lithium battery anodes. Energy Environ. Sci. 2011, 4, 56–72. [Google Scholar] [CrossRef]
- Choi, J.; Wang, J.; Matsumoto, T. Si Swarf Wrapped by Graphite Sheets for Li-Ion Battery Electrodes with Improved Overvoltage and Cyclability. J. Electrochem. Soc. 2021, 168, 020521. [Google Scholar] [CrossRef]
- Li, B.; Zhao, W.; Yang, Z.; Zhang, C.; Dang, F.; Liu, Y.; Jin, F.; Chen, X. A carbon-doped anatase TiO2-Based flexible silicon anode with high-performance and stability for flexible lithium-ion battery. J. Power Sources 2020, 466, 228339. [Google Scholar] [CrossRef]
- Zhao, L.; He, Y.-B.; Li, C.; Jiang, K.; Wang, P.; Ma, J.; Xia, H.; Chen, F.; He, Y.; Chen, Z.; et al. Compact Si/C anodes fabricated by simultaneously regulating the size and oxidation degree of Si for Li-ion batteries. J. Mater. Chem. A 2019, 7, 24356–24365. [Google Scholar] [CrossRef]
- Tian, X.; Xu, Q.; Cheng, L.; Meng, L.; Zhang, H.; Jia, X.; Bai, S.; Qin, Y. Enhancing the Performance of a Self-Standing Si/PCNF Anode by Optimizing the Porous Structure. ACS Appl. Mater. Inter. 2020, 12, 27219–27225. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Dai, Y.; Peng, D.; Huang, K. Synthesis of porous Si nanoparticles for high performances anode material in lithium-ion batteries. Mater. Res. Express 2021, 8, 025008. [Google Scholar] [CrossRef]
- Xie, Q.; Qu, S.; Zhao, P. A facile fabrication of micro/nano-sized silicon/carbon composite with a honeycomb structure as high-stability anodes for lithium-ion batteries. J. Electroanal. Chem. 2021, 884, 115074. [Google Scholar] [CrossRef]
- Hou, Y.-L.; Yang, Y.; Meng, W.-J.; Lei, B.-Y.; Ren, M.-X.; Yang, X.-X.; Wang, Y.-Q.; Zhao, D.-L. Core-shell structured Si@Cu nanoparticles encapsulated in carbon cages as high-performance lithium-ion battery anodes. J. Alloys Compd. 2021, 874, 159988. [Google Scholar] [CrossRef]
- Song, C.; Zhao, B.; Chen, S.; Ma, J.; Du, H. Nickel-assisted one-pot preparation of graphenic carbon matrices embedded with silicon nanoparticles as anode materials for lithium ion batteries. Carbon 2021, 179, 266–274. [Google Scholar] [CrossRef]
- Kwon, H.J.; Hwang, J.Y.; Shin, H.J.; Jeong, M.G.; Chung, K.Y.; Sun, Y.K.; Jung, H.G. Nano/Microstructured Silicon-Carbon Hybrid Composite Particles Fabricated with Corn Starch Biowaste as Anode Materials for Li-Ion Batteries. Nano Lett. 2020, 20, 625–635. [Google Scholar] [CrossRef]
- Di, F.; Wang, N.; Li, L.; Geng, X.; Yang, H.; Zhou, W.; Sun, C.; An, B. Coral-like porous composite material of silicon and carbon synthesized by using diatomite as self-template and precursor with a good performance as anode of lithium-ions battery. J. Alloys Compd. 2021, 854, 157253. [Google Scholar] [CrossRef]
- Xiang, B.; An, W.-L.; Fu, J.-J.; Mei, S.-X.; Guo, S.-G.; Zhang, X.-M.; Gao, B.; Chu, P.K. Graphene-encapsulated blackberry-like porous silicon nanospheres prepared by modest magnesiothermic reduction for high-performance lithium-ion battery anode. Rare Met. 2020, 40, 383–392. [Google Scholar] [CrossRef]
- Yi, X.; Zhang, F.; Wang, J.; Wang, S.; Tong, H.; An, T.; Yu, W.-J. Facile synthesis of N-C/Si@G nanocomposite as a high-performance anode material for Li-ion batteries. J. Alloys Compd. 2021, 872, 159716. [Google Scholar] [CrossRef]
- Phadatare, M.; Patil, R.; Blomquist, N.; Forsberg, S.; Ortegren, J.; Hummelgard, M.; Meshram, J.; Hernandez, G.; Brandell, D.; Leifer, K.; et al. Silicon-Nanographite Aerogel-Based Anodes for High Performance Lithium Ion Batteries. Sci. Rep. 2019, 9, 14621. [Google Scholar] [CrossRef]
- Zeng, Y.; Huang, Y.; Liu, N.; Wang, X.; Zhang, Y.; Guo, Y.; Wu, H.-H.; Chen, H.; Tang, X.; Zhang, Q. N-doped porous carbon nanofibers sheathed pumpkin-like Si/C composites as free-standing anodes for lithium-ion batteries. J. Energy Chem. 2021, 54, 727–735. [Google Scholar] [CrossRef]
- Ma, M.; Wang, H.; Li, X.; Peng, K.; Xiong, L.; Du, X. Free-standing SiOC/nitrogen-doped carbon fibers with highly capacitive Li storage. J. Eur. Ceram. Soc. 2020, 40, 5238–5246. [Google Scholar] [CrossRef]
- Mei, S.; Liu, Y.; Fu, J.; Guo, S.; Deng, J.; Peng, X.; Zhang, X.; Gao, B.; Huo, K.; Chu, P.K. Waste-glass-derived silicon/CNTs composite with strong Si-C covalent bonding for advanced anode materials in lithium-ion batteries. Appl. Surf. Sci. 2021, 563, 150280. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Yang, X.-X.; Ren, M.-X.; Lei, B.-Y.; Hou, Y.-L.; Meng, W.-J.; Zhao, D.-L. 3D CNTs networks enable core-shell structured Si@Ni nanoparticle anodes with enhanced reversible capacity and cyclic performance for lithium ion batteries. Int. J. Hydrogen Energy 2021, 46, 16179–16187. [Google Scholar] [CrossRef]
- Zhang, X.; Hayashida, R.; Tanaka, M.; Watanabe, T. Synthesis of carbon-coated silicon nanoparticles by induction thermal plasma for lithium ion battery. Powder Technol. 2020, 371, 26–36. [Google Scholar] [CrossRef]
- Wen, Y.; Rufford, T.E.; Chen, X.; Li, N.; Lyu, M.; Dai, L.; Wang, L. Nitrogen-doped Ti3C2Tx MXene electrodes for high-performance supercapacitors. Nano Energy 2017, 38, 368–376. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, F.; Tu, X.; Ma, X.; Xu, Q.; Dai, R.; Huang, X.; Yu, Y.; Lu, L. Facile Synthesis of MXene/Electrochemically Reduced Graphene Oxide Composites and Their Application for Electrochemical Sensing of Carbendazim. J. Electrochem. Soc. 2019, 166, B1673–B1680. [Google Scholar] [CrossRef]
- Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.-L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. MXene: A promising transition metal carbide anode for lithium-ion batteries. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Li, A.; Yu, Y.; Chen, X.; Song, H. Three-Dimensional Hierarchical Porous Structures Constructed by Two-Stage MXene-Wrapped Si Nanoparticles for Li-Ion Batteries. ACS Appl. Mater. Inter. 2020, 12, 48718–48728. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Chen, B.; Gu, F.; Zu, L.; Xu, M.; Feng, Y.; Wang, Z.; Zhang, H.; Zhang, C.; Yang, J. Ti3C2Tx MXene Nanosheets as a Robust and Conductive Tight on Si Anodes Significantly Enhance Electrochemical Lithium Storage Performance. ACS Nano 2020, 14, 5111–5120. [Google Scholar] [CrossRef]
- Jiang, M.; Jiang, M.; Gao, H.; Chen, J.; Liu, W.; Ma, Y.; Luo, W.; Yang, J. Comparison of Additives in Anode: The Case of Graphene, MXene, CNTs Integration with Silicon Inside Carbon Nanofibers. Acta Metall. Sin Engl. 2020, 34, 337–346. [Google Scholar] [CrossRef]
- Zhang, F.; Jia, Z.; Wang, C.; Feng, A.; Wang, K.; Hou, T.; Liu, J.; Zhang, Y.; Wu, G. Sandwich-like silicon/Ti3C2Tx MXene composite by electrostatic self-assembly for high performance lithium ion battery. Energy 2020, 195, 117047. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Z.; Xia, Y.; Wu, G.; Chen, C.; Wang, J.; Rao, P.; Dong, A. Facile electrostatic assembly of Si@MXene superstructures for enhanced lithium-ion storage. J. Colloid Interface Sci. 2020, 580, 68–76. [Google Scholar] [CrossRef]
- Gou, L.; Jing, W.; Li, Y.; Wang, M.; Hu, S.; Wang, H.; He, Y.-B. Lattice-Coupled Si/MXene Confined by Hard Carbon for Fast Sodium-Ion Conduction. ACS Appl. Energy Mater. 2021, 4, 7268–7277. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Zhou, Z. MXene-based materials for electrochemical energy storage. J. Energy Chem. 2018, 27, 73–85. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Bai, Z.; Lu, S. Recent Advances in Layered Ti3C2Tx MXene for Electrochemical Energy Storage. Small 2018, 14, 1703419. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Wang, M.; Wang, G.; Ma, Y.; Li, L.; Xiao, B.; Zheng, J. Self-assembly encapsulation of Si in N-doped reduced graphene oxide for use as a lithium ion battery anode with significantly enhanced electrochemical performance. Sustain. Energy Fuels 2019, 3, 1427–1438. [Google Scholar] [CrossRef]
- Kong, F.; He, X.; Liu, Q.; Qi, X.; Sun, D.; Zheng, Y.; Wang, R.; Bai, Y. Enhanced reversible Li-ion storage in Si@Ti3C2 MXene nanocomposite. Electrochem. Commun. 2018, 97, 16–21. [Google Scholar] [CrossRef]
- Xiao, W.; Qiu, Y.; Xu, Q.; Wang, J.; Xie, C.; Peng, J.; Hu, J.; Zhang, J.; Li, X. Building sandwich-like carbon coated Si@CNTs composites as high-performance anode materials for lithium-ion batteries. Electrochim. Acta 2020, 364, 137278. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, H.; Na, R.; Wang, D.; Shan, Z.; Tian, J. Facile preparation of void-buffered Si@TiO2/C microspheres for high-capacity lithium ion battery anodes. Electrochim. Acta 2020, 337, 135841. [Google Scholar] [CrossRef]
- Zheng, M.; Wu, S. A novel method for synthesizing Ti3C2Tx MXene nanosheets supported Si nanoparticles as lithium-ion batteries anode for electric vehicles applications. Mater. Lett. 2022, 311, 131570. [Google Scholar] [CrossRef]
- Muráth, S.; Varga, T.; Kukovecz, Á.; Kónya, Z.; Sipos, P.; Pálinkó, I.; Varga, G. Morphological aspects determine the catalytic activity of porous hydrocalumites: The role of the sacrificial templates. Mater. Today Chem. 2022, 23, 100682. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Feng, J. Flexible and Freestanding Silicon/MXene Composite Papers for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Inter. 2019, 11, 10004–10011. [Google Scholar] [CrossRef]
- Wang, T.; Ji, X.; Wu, F.; Yang, W.; Dai, X.; Xu, X.; Wang, J.; Guo, D.; Chen, M. Facile fabrication of a three-dimensional coral-like silicon nanostructure coated with a C/rGO double layer by using the magnesiothermic reduction of silica nanotubes for high-performance lithium-ion battery anodes. J. Alloys Compd. 2021, 863, 158569. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, Z.; Lai, J.; Chao, Y.; Yang, Y.; Zhou, P.; Li, Y.; Yang, W.; Xia, Z.; Guo, S. MXene/Si@SiOx@C Layer-by-Layer Superstructure with Autoadjustable Function for Superior Stable Lithium Storage. ACS Nano 2019, 13, 2167–2175. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Lu, D.; Guan, R.; Wang, J.; Bian, X. Si-based composite deriving from wok ash waste as high-performance anode for Li-ion battery. J. Alloys Compd. 2021, 858, 157680. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, C.; Wu, W.; Cai, Y.; Zhang, Y. Nodes-connected silicon-carbon nanofibrous hybrids anodes for lithium-ion batteries. Appl. Surf. Sci. 2021, 548, 148944. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Fuller, T.F. Analysis of the Lithium-Ion Insertion Silicon Composite Electrode/Separator/Lithium Foil Cell. J. Electrochem. Soc. 2011, 158, A859–A871. [Google Scholar] [CrossRef]
- Cheng, R.; Hu, T.; Zhang, H.; Wang, C.; Hu, M.; Yang, J.; Cui, C.; Guang, T.; Li, C.; Shi, C.; et al. Understanding the Lithium Storage Mechanism of Ti3C2Tx MXene. J. Phys. Chem. C 2018, 123, 1099–1109. [Google Scholar] [CrossRef]
- Li, H.; Lu, M.; Han, W.; Li, H.; Wu, Y.; Zhang, W.; Wang, J.; Zhang, B. Employing MXene as a matrix for loading amorphous Si generated upon lithiation towards enhanced lithium-ion storage. J. Energy Chem. 2019, 38, 50–54. [Google Scholar] [CrossRef]
- Wei, C.; Fei, H.; Tian, Y.; An, Y.; Tao, Y.; Li, Y.; Feng, J. Scalable construction of SiO/wrinkled MXene composite by a simple electrostatic self-assembly strategy as anode for high-energy lithium-ion batteries. Chin. Chem. Lett. 2020, 31, 980–983. [Google Scholar] [CrossRef]
- Zhou, H.; Cui, C.; Cheng, R.; Yang, J.; Wang, X. MXene Enables Stable Solid-Electrolyte Interphase for Si@MXene Composite with Enhanced Cycling Stability. ChemElectroChem 2021, 8, 3089–3094. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, D.; Ren, M.; Zhang, H.; Pan, L.; John Zhang, C.; Yang, J. Si@Ti3C2Tx with Si nanoparticles embedded in a 3D conductive network of crumpled Ti3C2Tx nanosheets for the anode of lithium-ion batteries with enhanced cycling performance. J. Alloys Compd. 2022, 892, 162037. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Huang, H.; Chu, X.; Xie, Y.; Xiong, D.; Yan, C.; Zhao, H.; Zhang, H.; Yang, W. Unraveling and Regulating Self-Discharge Behavior of Ti3C2Tx MXene-Based Supercapacitors. ACS Nano 2020, 14, 4916–4924. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, D.; Qian, Z.; Gu, X.; Yang, J.; Qian, Y. N-doped Ti3C2Tx MXene sheet-coated SiOx to boost lithium storage for lithium-ion batteries. Sci. China Mater. 2022, 66, 51–60. [Google Scholar] [CrossRef]
- Zhang, Z.; Ying, H.; Huang, P.; Zhang, S.; Zhang, Z.; Yang, T.; Han, W.-Q. Porous Si decorated on MXene as free-standing anodes for lithium-ion batteries with enhanced diffusion properties and mechanical stability. Chem. Eng. J. 2023, 451, 138785. [Google Scholar] [CrossRef]
- Shao, T.; Liu, J.; Gan, L.; Gong, Z.; Long, M. Yolk-shell Si@void@C composite with Chito-oligosaccharide as a C–N precursor for high capacity anode in lithium-ion batteries. J. Phys. Chem. Solids 2021, 152, 109965. [Google Scholar] [CrossRef]








| Materials | Specific Surface Area (m2 g−1) |
|---|---|
| NH2-Si1/MXene1 | 28.5 |
| NH2-Si3/MXene1 | 25.7 |
| NH2-Si5/MXene1 | 20.3 |
| Materials | Specific Current (A g−1) | Initial Capacity (mAh g−1) | Cycle Number | Reversible Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|
| Si@Ti3C2 MXene | 0.2 | 1195 | 150 | 188 | [32] |
| MXene and Si | 0.1 | 731 | 500 | 557.6 | [44] |
| NH2-Si/Ti3C2Tx | 0.3 | 1624.1 | 100 | 643.8 | [26] |
| SiO/wrinkled MXene | 0.5 | 1945 | 100 | 850 | [45] |
| Si@MXene | 0.2 | 1450 | 100 | 981 | [46] |
| Si@Ti3C2Tx | 0.5 | 4392 | 200 | 1000 | [47] |
| Si@MXene | 0.5 | 1238.5 | 150 | 1003.6 | [48] |
| SiOx@NTS | 0.5 | 1882.1 | 100 | 1141.3 | [49] |
| pSi/MXene | 0.1 | 2843.5 | 200 | 1039.3 | [50] |
| NH2-Si/MXene | 0.2 | 2395.1 | 100 | 1203.3 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Jiang, T.; Zhou, Y. Enhanced Lithium Storage Performance in Si/MXene Porous Composites. Inorganics 2023, 11, 279. https://doi.org/10.3390/inorganics11070279
Yang H, Jiang T, Zhou Y. Enhanced Lithium Storage Performance in Si/MXene Porous Composites. Inorganics. 2023; 11(7):279. https://doi.org/10.3390/inorganics11070279
Chicago/Turabian StyleYang, Hao, Tingting Jiang, and Yingke Zhou. 2023. "Enhanced Lithium Storage Performance in Si/MXene Porous Composites" Inorganics 11, no. 7: 279. https://doi.org/10.3390/inorganics11070279
APA StyleYang, H., Jiang, T., & Zhou, Y. (2023). Enhanced Lithium Storage Performance in Si/MXene Porous Composites. Inorganics, 11(7), 279. https://doi.org/10.3390/inorganics11070279