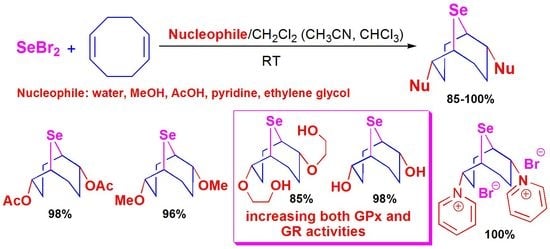
Transannular Selenocyclofunctionalization of 1,5-cyclooctadiene: The Antioxidant Properties of 9-selenabicyclo[3.3.1]nonane Derivatives and the Discovery of Increasing Both GPx and GR Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Evaluation
3. Materials and Methods
3.1. General Information
3.2. The Synthesis of Compound 3
3.3. The Synthesis of Compound 5
3.4. The Synthesis of Compound 6 (Modified Procedure)
3.5. The Synthesis of Compound 7
3.6. The Synthesis of Compound 8
3.7. Plant Material
3.8. Evaluation of Germinability and Mass of Seedlings
3.9. Determination of Protein Content
3.10. Determination of Glutathion Reductase Activity
3.11. Evaluation of Diene Conjugates
3.12. Statistics
3.13. X-ray Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serban, C.; Dragan, S. The relationship between inflammatory and oxidative stress biomarkers, atherosclerosis and rheumatic diseases. Curr. Pharm. Des. 2014, 20, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Afanasieva, G.A.; Chesnokova, N.P. On pathogenetic significance of activation of lipid peroxidation in the mechanisms of disturbance of blood rheologic properties in experimental intoxication induced by fraction FII of vaccinal EV strain of Y. pestis. Bull. Russ. Akad. Med. Sci. 2009, 2, 14–17. [Google Scholar]
- Lenardao, E.J.; Santi, C.; Sancineto, L. New Frontiers in Organoselenium Compounds; Springer International Publishing AG: Cham, Switzerland, 2018; 189p. [Google Scholar]
- Santi, C. Organoselenium Chemistry: Between Synthesis and Biochemistry; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014; 563p. [Google Scholar]
- Gandhil, U.H.; Nagaraja, T.P.; Prabhu, K.S. Selenoproteins and their role in oxidative stress and inflammation. Curr. Chem. Biol. 2013, 7, 65–73. [Google Scholar] [CrossRef]
- Mamgain, R.; Kostic, M.; Singh, F.V. Synthesis and Antioxidant Properties of Organoselenium Compounds. Curr. Med. Chem. 2023, 30, 2421–2448. [Google Scholar] [PubMed]
- Selvakumar, K.; Shah, P.; Singh, H.B.; Butcher, R.J. Synthesis, Structure, and Glutathione Peroxidase-Like Activity of Amino Acid Containing Ebselen Analogues and Diaryl Diselenides. Chem. Eur. J. 2011, 17, 12741–12755. [Google Scholar] [CrossRef]
- McNeil, N.M.R.; Press, D.J.; Mayder, D.M.; Garnica, P.; Doyle, L.M.; Back, T.G. Enhanced Glutathione Peroxidase Activity of Water-Soluble and Polyethylene Glycol-Supported Selenides, Related Spirodioxyselenuranes, and Pincer Selenuranes. J. Org. Chem. 2016, 81, 7884–7897. [Google Scholar] [CrossRef]
- Kumakura, F.; Mishra, B.; Priyadarsini, K.I.; Iwaoka, M. A Water-Soluble Cyclic Selenide with Enhanced Glutathione Peroxidase-Like Catalytic Activities. Eur. J. Org. Chem. 2010, 2010, 440–445. [Google Scholar] [CrossRef]
- Refaay, D.A.; Ahmed, D.M.; Mowafy, A.M.; Shaaban, S. Evaluation of novel multifunctional organoselenium compounds as potential cholinesterase inhibitors against Alzheimer’s disease. Med. Chem. Res. 2022, 31, 894–904. [Google Scholar] [CrossRef]
- Obieziurska-Fabisiak, M.; Pacuła-Miszewska, A.J.; Laskowska, A.; Ścianowski, J. Organoselenium compounds as antioxidants. Arkivoc 2023, 2023, 69–92. [Google Scholar] [CrossRef]
- Azad, G.K.; Tomar, R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014, 41, 4865–4879. [Google Scholar] [CrossRef]
- Plemenkov, V.V. Natural organic compounds of selenium. Butlerov Commun. 2006, 8, 1–5. [Google Scholar]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2180. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and organotellurium compounds: Toxicology and pharmacology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Therapeutic potential of selenium and tellurium compounds: Opportunities yet unrealized. Dalton Trans. 2012, 41, 6390–6395. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.; Koketsu, M. Recent developments in the synthesis of biologically relevant selenium-containing scaffolds. Coord. Chem. Rev. 2017, 339, 104–127. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V. New Methods for Preparation of Organoselenium and Organotellurium Compounds from Elemental Chalcogens. Russ. J. Org. Chem. 2003, 39, 1373–1380. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Musalova, M.V.; Amosova, S.V. Recent Advances in Organochalcogen Synthesis Based on Reactions of Chalcogen Halides with Alkynes and Alkenes. Curr. Org. Chem. 2016, 20, 136–145. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A. Selenium dihalides: New possibilities for the synthesis of selenium-containing heterocycles. Chem. Heterocycl. Compd. 2017, 53, 150–152. [Google Scholar] [CrossRef]
- Abakumov, G.A.; Piskunov, A.V.; Cherkasov, V.K.; Fedushkin, I.L.; Ananikov, V.P.; Eremin, D.B.; Gordeev, E.G.; Beletskaya, I.P.; Averin, A.D.; Bochkarev, M.N.; et al. Organoelement chemistry: Promising growth areas and challenges. Russ. Chem. Rev. 2018, 87, 393–507. [Google Scholar] [CrossRef]
- Musalov, M.V.; Yakimov, V.A.; Potapov, V.A.; Amosova, S.V.; Borodina, T.N.; Zinchenko, S.V. A novel methodology for the synthesis of condensed selenium heterocycles based on the annulation and annulation–methoxylation reactions of selenium dihalides. New J. Chem. 2019, 43, 18476–18483. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Maylyan, A.A.; Khabibulina, A.G.; Zinchenko, S.V.; Amosova, S.V. Selenium Dihalides Click Chemistry: Highly Efficient Stereoselective Addition to Alkynes and Evaluation of Glutathione Peroxidase-Like Activity of Bis(E-2-halovinyl) Selenides. Molecules 2022, 27, 1050. [Google Scholar] [CrossRef] [PubMed]
- Musalov, M.V.; Potapov, V.A.; Yakimov, V.A.; Musalova, M.V.; Maylyan, A.A.; Zinchenko, S.V.; Amosova, S.V. A Regioselective Synthesis of Novel Functionalized Organochalcogen Compounds by Chalcogenocyclofunctionalization Reactions Based on Chalcogen Halides and Natural Products. Molecules 2021, 26, 3729. [Google Scholar] [CrossRef] [PubMed]
- Potapov, V.A.; Musalov, M.V.; Amosova, S.V. Reactions of selenium dichloride and dibromide with unsaturated ethers. Annulation of 2,3-dihydro-1,4-oxaselenine to the benzene ring. Tetrahedron Lett. 2011, 52, 4606–4610. [Google Scholar] [CrossRef]
- Abramova, E.V.; Sterkhova, I.V.; Molokeev, M.S.; Potapov, V.A.; Amosova, S.V. First coordination compounds of SeBr2 with selenium ligands: X-ray structural determination. Mendeleev Commun. 2016, 26, 532–534. [Google Scholar] [CrossRef]
- Amosova, S.V.; Shagun, V.A.; Makhaeva, N.A.; Novokshonova, A.I.; Potapov, V.A. Quantum Chemical and Experimental Studies of an Unprecedented Reaction Pathway of Nucleophilic Substitution of 2-Bromomethyl-1,3-thiaselenole with 1,3-Benzothiazole-2-thiol Proceeding Stepwise at Three Different Centers of Seleniranium Intermediates. Molecules 2021, 26, 6685. [Google Scholar] [CrossRef]
- Wirth, T. Organoselenium Chemistry; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Potapov, V.A.; Musalov, M.V.; Khabibulina, A.G.; Maylyan, A.A.; Borodina, T.N.; Zinchenko, S.V.; Amosova, S.V. Regioselective One-Pot Synthesis of Novel Functionalized Organoselenium Compound by Bis-Alkoxyselenenylation of Alkenes with Selenium Dibromide and Alcohols. Inorganics 2022, 10, 239. [Google Scholar] [CrossRef]
- Converso, A.; Burow, K.; Marzinzik, A.; Sharpless, K.B.; Finn, M.G. 2,6-Dichloro-9-thiabicyclo[3.3.1]nonane: A privileged, bivalent scaffold for the display of nucleophilic components. J. Org. Chem. 2001, 66, 4386–4392. [Google Scholar] [CrossRef]
- Accurso, A.A.; Cho, S.-H.; Amin, A.; Potapov, V.A.; Amosova, S.V.; Finn, M.G. Thia-, Aza-, and Selena[3.3.1]bicyclononane Dichlorides: Rates vs. Internal Nucleophile in Anchimeric Assistance. J. Org. Chem. 2011, 76, 4392–4395. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Abramova, E.V.; Musalov, M.V.; Lyssenko, K.A.; Finn, M.G. 2,6-Dihalo-9-selenabicyclo[3.3.1]nonanes and their complexes with selenium dihalides: Synthesis and structural characterization. New J. Chem. 2015, 39, 8055–8059. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Amosova, S.V. Efficient Synthesis of a New Family of 2,6-Disulfanyl-9-selenabicyclo[3.3.1]nonanes. Molecules 2021, 26, 2849. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V. Triple-Click Chemistry of Selenium Dihalides: Catalytic Regioselective and Highly Efficient Synthesis of Bis-1,2,3-Triazole Derivatives of 9-Selenabicyclo[3.3.1]nonane. Catalysts 2022, 12, 1032. [Google Scholar] [CrossRef]
- Yurieva, O.V.; Dubrovina, V.I.; Potapov, V.A.; Musalov, M.V.; Starovoitova, T.P.; Ivanova, T.A.; Gromova, A.V.; Shkaruba, T.T.; Balakhonov, S.V. Effect of Synthetic Organoselenium Drug on the Degree of Pathological Changes in the Organs of White Mice Immunized with Tularemia and Brucellosis Vaccines. Bull. Exp. Biol. Med. 2019, 168, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Dubrovina, V.I.; Starovoitova, T.P.; Yur’eva, O.V.; Pyatidesyatnikova, A.B.; Potapov, V.A.; Musalov, M.V.; Balakhonov, S.V. Effect of a Synthetic Organoselenium Compound on Post-Vaccination Immunopoiesis in the Red Bone Marrow and Cell Composition of Peripheral Blood of White Mice Vaccinated with Yersinia pestis EV. Bull. Exp. Biol. Med. 2021, 171, 651–655. [Google Scholar] [CrossRef]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868–883. [Google Scholar] [CrossRef] [Green Version]
- Mugesh, G.; Panda, A.; Singh, H.B.; Punekar, N.S.; Butcher, R.J. Glutathione Peroxidase-like Antioxidant Activity of Diaryl Diselenides: A Mechanistic Study. J. Am. Chem. Soc. 2001, 123, 839–850. [Google Scholar] [CrossRef]
- Geng, Z.; Finn, M.G. Fragmentable Polycationic Materials Based on Anchimeric Assistance. Chem. Mater. 2016, 28, 146–152. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione reductase and glutathione: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef]
- Dorofeev, N.V.; Bojarkin, E.V.; Peshkova, A.A. Factors Defining Field Germination of Oilseed Radish Seeds. J. Stress Phys. Biochem. 2013, 9, 159–168. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nigmatullina, L.R.; Rumyantseva, N.I.; Kostyukova, Y.A. The effect of D,L-buthionine-S,R-sulfoximine on the ratio of glutathione forms and the growth of Tatar buckwheat calli. Ontogenesis 2014, 45, 50–62. [Google Scholar] [CrossRef]
- Placer, Z. Lip peroxidation systeme im biologischen material. Nahrung 1968, 12, 679–684. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]














| Compounds | Reference | Compounds | Reference |
|---|---|---|---|
 1 |  |  6 | Na2SeO3 |
 3 |  7 | ||
 5 |  8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musalov, M.V.; Kapustina, I.S.; Spiridonova, E.V.; Ozolina, N.V.; Amosova, S.V.; Borodina, T.N.; Potapov, V.A. Transannular Selenocyclofunctionalization of 1,5-cyclooctadiene: The Antioxidant Properties of 9-selenabicyclo[3.3.1]nonane Derivatives and the Discovery of Increasing Both GPx and GR Activities. Inorganics 2023, 11, 304. https://doi.org/10.3390/inorganics11070304
Musalov MV, Kapustina IS, Spiridonova EV, Ozolina NV, Amosova SV, Borodina TN, Potapov VA. Transannular Selenocyclofunctionalization of 1,5-cyclooctadiene: The Antioxidant Properties of 9-selenabicyclo[3.3.1]nonane Derivatives and the Discovery of Increasing Both GPx and GR Activities. Inorganics. 2023; 11(7):304. https://doi.org/10.3390/inorganics11070304
Chicago/Turabian StyleMusalov, Maxim V., Irina S. Kapustina, Ekaterina V. Spiridonova, Natalya V. Ozolina, Svetlana V. Amosova, Tatyana N. Borodina, and Vladimir A. Potapov. 2023. "Transannular Selenocyclofunctionalization of 1,5-cyclooctadiene: The Antioxidant Properties of 9-selenabicyclo[3.3.1]nonane Derivatives and the Discovery of Increasing Both GPx and GR Activities" Inorganics 11, no. 7: 304. https://doi.org/10.3390/inorganics11070304
APA StyleMusalov, M. V., Kapustina, I. S., Spiridonova, E. V., Ozolina, N. V., Amosova, S. V., Borodina, T. N., & Potapov, V. A. (2023). Transannular Selenocyclofunctionalization of 1,5-cyclooctadiene: The Antioxidant Properties of 9-selenabicyclo[3.3.1]nonane Derivatives and the Discovery of Increasing Both GPx and GR Activities. Inorganics, 11(7), 304. https://doi.org/10.3390/inorganics11070304