Physicochemical Fundamentals of the Synthesis of a Cu@BN Composite Consisting of Nanosized Copper Enclosed in a Boron Nitride Matrix
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Compounds
2.1.1. [CuII(N2H4)3][B10H10]·nH2O (I·nH2O) and [CuII(NH3)4][B10H10]·nH2O (II·nH2O)
2.1.2. Samples I900 and II900
2.2. Methods of Investigation
3. Results and Discussion
3.1. Synthesis of Precursor Compounds [CuII(N2H4)3][B10H10]·nH2O (I·nH2O) and [CuII(NH3)4][B10H10]·nH2O (II·nH2O)
3.2. TGA and IR Data Obtained for Compounds I·nH2O and II·nH2O
- (1)
- A broad endothermic effect with a maximum at 130 °C accompanied by a sample weight loss of 5.47% corresponds to the partial removal of solvated water molecules. This is also evidenced by the data from the IR spectrum of sample I135, in which there is a decrease in the intensity of the broadened band of stretching vibrations ν(OH)H2O at about 3400 cm−1 and the band of bending vibrations δ(HOH) at 1620 cm−1, observed in the spectrum of sample I (Figure 3).
- (2)
- The exothermic effect with a maximum at 213 °C corresponds to the exothermic decomposition of hydrazine molecules in the sample and its complete hydration, while the weight loss of the sample is 4.23%. The decomposition of hydrazine molecules is evidenced by the data from the IR spectrum of sample I220, in which there are no ν(NH)N2H4 stretching vibration bands. In addition, when the sample is heated to 220 °C, in the presence of hydrazine molecules exhibiting reducing properties, the reduction of Cu(II) to Cu(I) and the formation of a Cu(I) complex with the [B10H10]2− anion as a ligand is observed. Thus, in the IR spectrum of sample I220, along with the bands of stretching vibrations of free BH bonds ν(BH), a new broadened band appears in the range of 2300–2150 cm−1, which is characteristic of stretching vibrations of the BH groups involved in the three-center CuHB bonds, ν(BH)CuHB (Figure 3). Note that the pattern of the IR spectrum of sample I220 in the region of ν(BH) is similar to the spectrum of complex {Cu2[B10H10]}n (Figure 1).
- (3)
- The heating of sample I·nH2O above 300 °C leads to the destruction of the closo-decaborate anion and is accompanied by an exothermic effect with a maximum at 323 °C. At this stage, apparently, the processes associated with the reduction of Cu(I)→Cu(0) come to an end. This assumption is not contradicted by the change in the TG curve and the absence of pronounced thermal effects, which may be associated with the oxidation of Cu(0) by trace amounts of oxygen in the gas used.
3.3. Thermal Reduction of Compounds I·nH2O and II·nH2O
3.4. Morphology of Samples I900 and II900
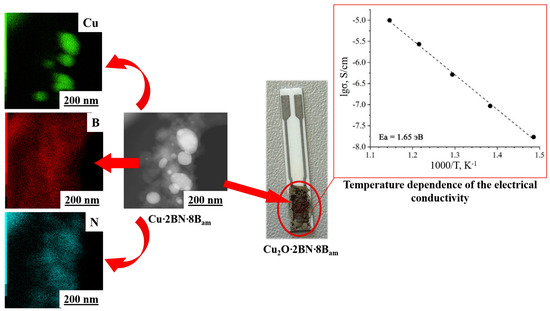
3.5. Electrical Conductivity of Sample I900
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosmane, N.S.; Eagling, R. (Eds.) Handbook of Boron Science with Applications in Organometallics, Catalysis, Materials and Medicine; World Scientific Publishing Europe: London, UK, 2018; p. 1056. [Google Scholar]
- Hey-Hawkins, E.; Viñas Teixidor, C. (Eds.) Boron-Based Compounds: Potential and Emerging Applications in Medicine; John Wiley & Sons Ltd.: Oxford, UK, 2018; p. 496. [Google Scholar]
- Fink, K.; Uchman, M. Boron cluster compounds as new chemical leads for antimicrobial therapy. Coord. Chem. Rev. 2021, 431, 213684. [Google Scholar] [CrossRef]
- Imperio, D.; Panza, L. Sweet Boron: Boron-Containing Sugar Derivatives as Potential Agents for Boron Neutron Capture Therapy. Symmetry 2022, 14, 182. [Google Scholar] [CrossRef]
- Novopashina, D.S.; Vorobyeva, M.A.; Venyaminova, A. Recent Advances in the Synthesis of High Boron-Loaded Nucleic Acids for BNCT. Front. Chem. 2021, 9, 619052. [Google Scholar] [CrossRef]
- Ali, F.; Hosmane, N.S.; Zhu, Y. Boron Chemistry for Medical Applications. Molecules 2020, 25, 828. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Ishimura, M.; Ohta, Y.; Takenaka, H.; Kawabata, S.; Kirihata, M. Dodecaborate Conjugates Targeting Tumor Cell Overexpressing Translocator Protein for Boron Neutron Capture Therapy. ACS Med. Chem. Lett. 2022, 13, 50–54. [Google Scholar] [CrossRef]
- Rao, M.H.; Muralidharan, K. Syntheses, characterization and energetic properties of closo-(B12H12)2− salts of imidazolium derivatives. Dalton Trans. 2013, 42, 8854–8860. [Google Scholar]
- Zhang, Z.; Zhao, Z.; Wang, B.; Zhang, J. Boron based hypergolic ionic liquids: A review. Green Energy Environ. 2021, 6, 794–822. [Google Scholar] [CrossRef]
- Jiao, N.; Zhang, Y.; Liu, L.; Shreeve, J.M.; Zhang, S. Hypergolic fuels based on water-stable borohydride cluster anions with ultralow ignition delay times. J. Mater. Chem. A 2017, 5, 13341–13346. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Li, Z.; Jiao, N.; Liu, L.; Zhang, S. B12H122–-Based Metal (Cu2+, Ni2+, Zn2+) Complexes as Hypergolic Fuels with Superior Hypergolicity. Eur. J. Inorg. Chem. 2018, 2018, 981–986. [Google Scholar] [CrossRef]
- Mukherjee, S.; Thilagar, P. Boron clusters in luminescent materials. Chem. Commun. 2016, 52, 1070–1093. [Google Scholar] [CrossRef]
- Núñez, R.; Tarrés, M.; Ferrer-Ugalde, A.; Biani, F.F.; Teixidor, F. Electrochemistry and Photoluminescence of Icosahedral Carboranes, Boranes, Metallacarboranes, and Their Derivatives. Chem. Rev. 2016, 116, 14307–14378. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Flores, B.M.; Cabrera-González, J.; Viñas, C.; Chávez-Reyes, A.; Dias, H.V.R.; Jiménez-Pérez, V.M.; Núñez, R. Organotin Dyes Bearing Anionic Boron Clusters as Cell-Staining Fluorescent Probes. Chem. Eur. J. 2018, 24, 5601–5612. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-González, J.; Chaari, M.; Teixidor, F.; Viñas, C.; Núñez, R. Blue Emitting Star-Shaped and Octasilsesquioxane-Based Polyanions Bearing Boron Clusters. Photophysical and Thermal Properties. Molecules 2020, 25, 1210. [Google Scholar] [PubMed]
- Corona-López, M.M.; Muñoz-Flores, B.M.; Chaari, M.; Nuñez, R.; Jiménez-Pérez, V.M. Far-Red and Near-Infrared Boron Schiff Bases (BOSCHIBAs) Dyes Bearing Anionic Boron Clusters. Eur. J. Inorg. Chem. 2021, 2021, 2047–2054. [Google Scholar] [CrossRef]
- Oyelade, A.; Yost, A.J.; Benker, N.; Dong, B.; Knight, S.; Schubert, M.; Dowben, P.A.; Kelber, J.A. Composition-dependent charge transport in boron carbides alloyed with aromatics: Plasma enhanced chemical vapor deposition aniline/ortho-carborane films. Langmuir 2018, 34, 12007–12016. [Google Scholar] [CrossRef]
- Oyelade, A.; Osonkie, A.; Yost, A.J.; Benker, N.; Dowben, P.A.; Kelber, J.A. Optical, electronic and visible-range photo-electronic properties of boron carbide-indole films. J. Phys. D 2020, 53, 355101. [Google Scholar] [CrossRef]
- Sharapov, V.M. Discharge chamber plasma-chemical conditioning in magnetic confinement fusion devices (Review). Phys. Atom. Nucl. 2021, 84, 1266–1271. [Google Scholar] [CrossRef]
- Yan, D.; Chen, J.; Zhang, Y.; Gou, Y. Preparation of novel carborane-containing boron carbide precursor and its derived ceramic hollow microsphere. Ceram. Int. 2022, 48, 18392–18400. [Google Scholar] [CrossRef]
- Filonenko, V.P.; Zinin, P.V.; Zibrov, I.P.; Anokhin, A.S.; Kukueva, E.V.; Lyapin, S.G.; Fominski, V.Y. Synthesis of star-shaped boron carbide micro-crystallites under high pressure and high temperatures. Crystals 2018, 8, 448. [Google Scholar] [CrossRef]
- Pavlov, I.S.; Ivanova, A.G.; Filonenko, V.P.; Zibrov, I.P.; Voloshin, A.E.; Zinin, P.V.; Vasiliev, A.L. The rhombic hexecontahedronboron carbide microcrystals—Crystal structure analysis. Scr. Mater. 2023, 222, 115023. [Google Scholar] [CrossRef]
- Bagramov, R.H.; Filonenko, V.P.; Zibrov, I.P.; Skryleva, E.A.; Nikolaev, A.V.; Pasternak, D.G.; Vlasov, I.I. Highly boron-doped graphite and diamond synthesized from adamantane and ortho-carborane under high pressure. Materialia 2022, 21, 101274. [Google Scholar] [CrossRef]
- Malinina, E.A.; Myshletsov, I.I.; Buzanov, G.A.; Kubasov, A.S.; Kozerozhets, I.V.; Goeva, L.V.; Nikiforova, S.E.; Avdeeva, V.V.; Zhizhin, K.Y.; Kuznetsov, N.T. A New Approach to the Synthesis of Nanocrystalline Cobalt Boride in the Course of the Thermal Decomposition of Cobalt Complexes [Co(DMF)6]2+ with Boron Cluster Anions. Molecules 2023, 28, 453. [Google Scholar] [CrossRef] [PubMed]
- Avdeeva, V.V.; Polyakova, I.N.; Vologzhanina, A.V.; Goeva, L.V.; Buzanov, G.A.; Generalova, N.B.; Malinina, E.A.; Zhizhin, K.Y.; Kuznetsov, N.T. [Co(solv)6][B10H10] (solv = DMF and DMSO) for low-temperature synthesis of borides. Russ. J. Inorg. Chem. 2016, 61, 1125–1134. [Google Scholar] [CrossRef]
- Malinina, E.A.; Goeva, L.V.; Buzanov, G.A.; Avdeeva, V.V.; Kuznetsov, N.T.; Retivov, V.M. Synthesis and Thermal Reduction of Complexes [NiLn][B10H10] (L = DMF, H2O, n = 6; L = N2H4, n = 3): Formation of Solid Solutions Ni3C1–xBx. Russ. J. Inorg. Chem. 2020, 65, 126–132. [Google Scholar] [CrossRef]
- Malinina, E.A.; Goeva, L.V.; Buzanov, G.A.; Avdeeva, V.V.; Efimov, N.N.; Kozerozhets, I.V.; Kuznetsov, N.T. Synthesis and Physicochemical Properties of Binary Cobalt(II) Borides. Thermal Reduction of Precursor Complexes [CoLn][B10H10] (L = H2O, n = 6; N2H4, n = 3). Russ. J. Inorg. Chem. 2019, 64, 1325–1334. [Google Scholar] [CrossRef]
- Okoye, P.C.; Azi, S.O.; Qahtan, T.F.; Owolabi, T.O.; Sakeh, T.A. Synthesis, properties, and applications of doped and undoped CuO and Cu2O nanomaterials. Mater. Today Chem. 2023, 30, 101513. [Google Scholar] [CrossRef]
- Crovetto, A.; Unold, T.; Zakutayev, A. Is Cu3-xP a Semiconductor, a Metal, or a Semimetal? Chem. Mater. 2023, 35, 1259–1272. [Google Scholar] [CrossRef]
- Crovetto, A.; Hempel, H.; Rusu, M.; Choubrac, L.; Kojda, D.; Habicht, K.; Unold, T. Water Adsorption Enhances Electrical Conductivity in Transparent P-Type CuI. ACS Appl. Mater. Interfaces 2020, 12, 48741–48747. [Google Scholar] [CrossRef]
- Basha, B.; Jacob, J.; Tanveer, Z.; Ali, A.; Amin, N.; Javaid, K.; Ikham, S. Effect of source to the substrate distance on thermoelectric properties of Copper Nitride thin films grown by thermal evaporation method. J. Mater. Res. Technol. 2023, 25, 265–272. [Google Scholar] [CrossRef]
- Moletti, A.; Coluccini, C.; Pasini, D.; Taglietti, A. A chiral probe for the detection of Cu(II) by UV, CD and emission spectroscopies. Dalton Trans. 2007, 16, 1588–1592. [Google Scholar] [CrossRef]
- Liu, A.; Kin, M.-G.; Kim, J.; Noh, Y.-Y. Engineering Copper Iodide (CuI) for Multifunctional p-Type Transparent Semiconductors and Conductors. Adv. Sci. 2021, 8, 2100546. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.K.; Polity, A.; Reppin, D.; Becker, M.; Hering, P.; Klar, P.J.; Sander, T.; Reindl, C.; Benz, J.; Eickhoff, M.; et al. Binary copper oxide semiconductors: From materials towards devices. Phys. Stat. Solidi B 2012, 249, 1487–1509. [Google Scholar] [CrossRef]
- Wang, Y.; Pierson, J.F. Binary copper oxides as photovoltaic absorbers: Recent progress in materials and applications. J. Phys. D Appl. Phys. 2021, 54, 263002. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, K.; Yue, Z.; Wang, Y.; Song, Q.; Li, J.; Guan, M.; Xu, Q.; Qiu, P.; Zhu, H.; et al. Are Cu2Te-Based Compounds Excellent Thermoelectric Materials? Adv. Mater. 2019, 31, 1903480. [Google Scholar] [CrossRef]
- Jong, U.-G.; Ri, C.-H.; Pak, C.-J.; Kim, C.-H.; Cottenier, S.; Yu, C.-J. Metal Phosphide CuP2 as a Promising Thermoelectric Material: An Insight from a First-Principles Study. New J. Chem. 2021, 45, 21569–21576. [Google Scholar] [CrossRef]
- Pöhls, J.-H.; Faghaninia, A.; Petretto, G.; Aydemir, U.; Ricci, F.; Li, G.; Wood, M.; Ohno, S.; Hautier, G.; Snyder, A. Metal Phosphides as Potential Thermoelectric Materials. J. Mater. Chem. C 2017, 5, 12441–12456. [Google Scholar] [CrossRef]
- Minami, T.; Nishi, Y.; Miyata, T. Efficiency Enhancement Using a Zn1-xGex-O Thin Film as an n-Type Window Layer in Cu2O-based Heterojunction Solar Cells. Appl. Phys. Express 2016, 9, 052301. [Google Scholar] [CrossRef]
- Han, B.; Liu, W.; Duan, J.; Chen, W.; Li, D.; Xu, X.; Chang, Q.; Yang, Z.; Wang, Y. In Situ Gravimetric Probing of Copper Sulfide Formation on the Counter Electrode for Quantum Dot Sensitized Solar Cells. J. Phys. Chem. C 2023, 127, 10833–10844. [Google Scholar] [CrossRef]
- Zakutayev, A.; Caskey, C.M.; Fioretti, A.N.; Ginley, D.S.; Vidal, J.; Stevanović, V.; Tea, E.; Lany, S. Defect Tolerant Semiconductors for Solar Energy Conversion. J. Phys. Chem. Lett. 2014, 5, 1117–1125. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Ebrahim, S.E. Recent advances in nano-semiconductors photocatalysis for degrading organic contaminants and microbial disinfection in wastewater: A comprehensive review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100666. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, P.; Cui, Y.; Fu, X.; Wang, Y. Recent progress in copper-based inorganic nanostructure photocatalysts: Properties, synthesis and photocatalysis applications. Mater. Today Sustain. 2023, 21, 100276. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Kubasov, A.S.; Korolenko, S.E.; Goeva, L.V.; Malinina, E.A.; Kuznetsov, N.T. Synthesis and Structures of Copper and Thallium(I) Coordination Compounds [Cu2[B10H10]]n and Tl2[B10H10] with the closo-Decaborate Anion. Russ. J. Inorg. Chem. 2022, 67, 628–635. [Google Scholar] [CrossRef]
- Pokropivny, V.V.; Smolyar, A.S.; Ovsiannikova, L.I.; Pokropivny, A.V.; Kuts, V.A.; Lyashenko, V.I.; Nesterenko, Y.V. Fluid synthesis and structure of a new polymorphic modification of boron nitride. Phys. Solid State 2013, 55, 878–884. [Google Scholar] [CrossRef]
- Badakhsh, A.; Cha, J.; Park, Y.; Lee, Y.-J.; Jeong, H.; Kim, Y.; Sohn, H.; Nam, S.W.; Yoon, C.W.; Park, C.W.; et al. Autothermal recirculating reactor (ARR) with Cu-BN composite as a stable reactor material for sustainable hydrogen release from ammonia. J. Power Sources 2021, 506, 230081. [Google Scholar] [CrossRef]
- Hepel, M.; Tannatoli, T.; Baxter, C.; Stephenson, R. Composite Films of Copper/Boron Nitride and Nickel/Boron Nitride. MRS Online Proc. Libr. 1996, 451, 481–488. [Google Scholar] [CrossRef]
- Chusong, E.; Kansuwan, P.; Khosakul, R.; Ohtake, N.; Wila, P.; Tosangthum, N.; Vetayanugul, B.; Tongsri, R. Sintered 316L/Cu/h-BN composites. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1137, 012036. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, Q.; Kang, X.; Liu, H.; Qian, Q.; Ma, J.; Zhang, Z.; Yanga, G.; Han, B. Design of a Cu(I)/C-doped boron nitride electrocatalyst for efficient conversion of CO2 into acetic acid. Green Chem. 2017, 19, 2086–2091. [Google Scholar] [CrossRef]
- Xu, S.; Niu, M.; Zhao, G.; Ming, S.; Li, X.; Zhu, Q.; Ding, L.-X.; Kim, M.; Alothman, A.A.; Mushab, M.S.S.; et al. Size control and electronic manipulation of Ru catalyst over B, N co-doped carbon network for high-performance hydrogen evolution reaction. Nano Res. 2023, 16, 6212–6219. [Google Scholar] [CrossRef]















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malinina, E.A.; Myshletsov, I.I.; Buzanov, G.A.; Kozerozhets, I.V.; Simonenko, N.P.; Simonenko, T.L.; Nikiforova, S.E.; Avdeeva, V.V.; Zhizhin, K.Y.; Kuznetsov, N.T. Physicochemical Fundamentals of the Synthesis of a Cu@BN Composite Consisting of Nanosized Copper Enclosed in a Boron Nitride Matrix. Inorganics 2023, 11, 345. https://doi.org/10.3390/inorganics11080345
Malinina EA, Myshletsov II, Buzanov GA, Kozerozhets IV, Simonenko NP, Simonenko TL, Nikiforova SE, Avdeeva VV, Zhizhin KY, Kuznetsov NT. Physicochemical Fundamentals of the Synthesis of a Cu@BN Composite Consisting of Nanosized Copper Enclosed in a Boron Nitride Matrix. Inorganics. 2023; 11(8):345. https://doi.org/10.3390/inorganics11080345
Chicago/Turabian StyleMalinina, Elena A., Ivan I. Myshletsov, Grigorii A. Buzanov, Irina V. Kozerozhets, Nikolay P. Simonenko, Tatiana L. Simonenko, Svetlana E. Nikiforova, Varvara V. Avdeeva, Konstantin Yu. Zhizhin, and Nikolay T. Kuznetsov. 2023. "Physicochemical Fundamentals of the Synthesis of a Cu@BN Composite Consisting of Nanosized Copper Enclosed in a Boron Nitride Matrix" Inorganics 11, no. 8: 345. https://doi.org/10.3390/inorganics11080345
APA StyleMalinina, E. A., Myshletsov, I. I., Buzanov, G. A., Kozerozhets, I. V., Simonenko, N. P., Simonenko, T. L., Nikiforova, S. E., Avdeeva, V. V., Zhizhin, K. Y., & Kuznetsov, N. T. (2023). Physicochemical Fundamentals of the Synthesis of a Cu@BN Composite Consisting of Nanosized Copper Enclosed in a Boron Nitride Matrix. Inorganics, 11(8), 345. https://doi.org/10.3390/inorganics11080345