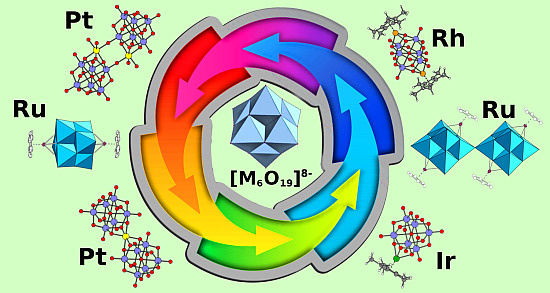
Polyoxoniobates and Polyoxotantalates as Ligands—Revisited
Abstract
:1. Introduction
2. Reactivity of [M6O19]8− towards Noble Metal Complexes
2.1. Reaction [M6O19]8− with {Cp*Rh}2+ and {Cp*Ir}2+


2.2. Complexation of [Ta6O19]8− with {(C6H6)Ru}2+

2[{(C6H6)Ru}Ta6O19H]5− = [{(C6H6)RuTa6O18}2(μ-O)]10− + H2O

2.3. Reaction [Nb6O19]8− and Pt4+

3. Reactivity of Heteropolyniobates towards Noble Metal Complexes
3.1. Reaction [(OH)TeNb5O19]6− with {Cp*Rh}2+ and {Cp*Ir}2+

3.2. Reaction [(OH)TeNb5O19]6− with {(C6H6)Ru}2+

3.3. Reaction [SiNb12O40]16− with {(C6H6)Ru}2+
4. Analytical Tools to Study Solution Speciation in Nb and Ta POM Chemistry
4.1. {(C6H6)Ru}2+/[Ta6O19]8− System—Case Study

2[{(C6H6)Ru}2Ta6O19]4− + 3OH− + = 2[{(C6H6)Ru}Ta6O19]6− + [(C6H6)2Ru2(μ-OH)3]+

5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Livage, J. Synthesis of polyoxovanadates via “chimie douce”. Coord. Chem. Rev. 1998, 178–189, 999–1018. [Google Scholar] [CrossRef]
- Hayashi, Y. Hetero and lacunary polyoxovanadate chemistry: Synthesis, reactivity and structural aspects. Coord. Chem. Rev. 2011, 255, 2270–2280. [Google Scholar] [CrossRef]
- Müller, A.; Sessoli, R.; Krickemeyer, E.; Bögge, H.; Meyer, J.; Gatteschi, D.; Pardi, L.; Westphal, J.; Hovemeier, K.; Rohlfing, R.; et al. Polyoxovanadates: High-Nuclearity Spin Clusters with Interesting Host–Guest Systems and Different Electron Populations. Synthesis, Spin Organization, Magnetochemistry, and Spectroscopic Studies. Inorg. Chem. 1997, 36, 5239–5250. [Google Scholar] [CrossRef]
- Breen, J.M.; Zhang, L.; Clement, R.; Schmitt, W. Hybrid Polyoxovanadates: Anion-Influenced Formation of Nanoscopic Cages and Supramolecular Assemblies of Asymmetric Clusters. Inorg. Chem. 2012, 51, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Schmitt, W. From Platonic Templates to Archimedean Solids: Successive Construction of Nanoscopic {V16As8}, {V16As10}, {V20As8}, and {V24As8} Polyoxovanadate Cages. J. Am. Chem. Soc. 2011, 133, 11240–11248. [Google Scholar] [CrossRef] [PubMed]
- Gatteschi, D.; Sessoli, R.; Müller, A.; Kögerler, P. Polyoxometalate chemistry: A source for unusual spin topologies. In Polyoxometalate Chemistry; Pope, M.T., Müller, A., Eds.; Kluwer: Dordrecht, The Netherlands, 2001; p. 319. [Google Scholar]
- Choi, J.; Sanderson, L.A.W.; Musfeldt, J.L.; Ellern, A.; Kögerler, P. Optical properties of the molecule-based magnet K6[V15As6O42(H2O)]·8H2O. Phys. Rev. B 2003, 68, 064412. [Google Scholar] [CrossRef]
- Gatteschi, D.; Pardi, L.; Barra, A.L.; Müller, A. Polyoxovanadates: The Missing Link between Simple Paramagnets and Bulk Magnets? Mol. Eng. 1993, 3, 157–169. [Google Scholar] [CrossRef]
- Nymann, M. Polyoxoniobate chemistry in the 21st century. Dalton Trans. 2011, 40, 8049–8058. [Google Scholar] [CrossRef] [PubMed]
- Kato, R.; Kobayashi, A.; Sasaki, Yu. 1:14 Heteropolyvanadate of phosphorus: preparation and structure. J. Am. Chem. Soc. 1980, 102, 6571–6572. [Google Scholar] [CrossRef]
- Kato, R.; Kobayashi, A.; Sasaki, Yu. The heteropolyvanadate of phosphorus. Crystallographic and NMR studies. Inorg. Chem. 1982, 21, 240–246. [Google Scholar] [CrossRef]
- Nomiya, K.; Kato, K.; Miwa, M. Preparation and spectrochemical properties of soluble vanadophosphate polyanions with bicapped-Keggin structure. Polyhedron 1986, 5, 811–813. [Google Scholar] [CrossRef]
- Khan, M.I.; Zubieta, J.; Toscano, P. Protonation sites in a heteropolyvanadate of phosphorus: X-ray crystal structure of (Me3NH)4(NH4)[H4PV14O42]. Inorg. Chim. Acta 1992, 193, 17–20. [Google Scholar] [CrossRef]
- Nakamura, S.; Yamawaki, T.; Kusaka, K.; Otsuka, T.; Ozeki, T. Hydrogen-bond Networks Involving Protonated Bicapped-Keggin Tetradecavanadophosphate Anions. J. Clust. Sci. 2006, 17, 245–256. [Google Scholar] [CrossRef]
- Grabau, M.; Forster, J.; Heussner, K.; Streb, C. Synthesis and Theoretical Hirshfeld Analysis of a Supramolecular Heteropolyoxovanadate Architecture. Eur. J. Inorg. Chem. 2011, 1719–1724. [Google Scholar] [CrossRef]
- Fang, X.; Kögerler, P.; Speldrich, M.; Schilder, H.; Luban, M. A polyoxometalate-based single-molecule magnet with an S = 21/2 ground state. Chem. Commun. 2012, 48, 1218–1220. [Google Scholar] [CrossRef]
- Monakhov, K.Yu.; Linnenberg, O.; Kozłowski, P.; van Leusen, J.; Besson, C.; Secker, T.; Ellern, A.; López, X.; Poblet, J.M.; Kögerler, P. Supramolecular Recognition Influences Magnetism in [X@HVIV8VV14O54]6− Self-Assemblies with Symmetry-Breaking Guest Anions. Chem. Eur. J. 2015, 21, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Dale, B.W.; Pope, M.T. The heteropoly-12-niobomanganate(IV) anion. J. Chem. Soc. Chem. Commun. 1967, 792–792. [Google Scholar]
- Flynn, C.M., Jr.; Stucky, G.D. Heteropolyniobate complexes of manganese(IV) and nickel(IV). Inorg. Chem. 1969, 8, 332–334. [Google Scholar] [CrossRef]
- Flynn, C.M., Jr.; Stucky, G.D. Crystal structure of sodium 12-niobomanganate(IV),Na12MnNb12O38·50H2O. Inorg. Chem. 1969, 8, 335–344. [Google Scholar] [CrossRef]
- Kheddar, N.; Spinner, B. Constitution et domaines de stabilite des oxaloniobates en solution aqueuse. Bull. Soc. Chim. Fr. 1972, 2, 502–506. (In French) [Google Scholar]
- Marty, A.; Abdmeziem, K.; Spinner, B. Une nouvelle condensation pour des sels du niobium V: les nonaniobates de tetramethyl et de tetraethylammonium. Comptes Rendus C. 1976, 283, 285–288. [Google Scholar]
- Marty, A.; Abdmeziem, K.; Spinner, B. De noveaux isopolyanions du niobium V: comportement en solution aqueuse des nonaniobates de tetramethyl et tetraethylammonium. Bull. Soc. Chim. Fr. 1977, 3–4, 231–238. (In French) [Google Scholar]
- Goiffon, A.; Philippot, E.; Maurin, M. Structure cristalline du niobate 7/6 de sodium (Na7)(H3O)Nb6O19·14H2O. Rev. Chim. Miner. 1980, 17, 466–476. [Google Scholar]
- Graeber, E.J.; Morosin, B. The molecular configuration of the decaniobate ion (Nb10O286−). Acta Cryst. 1977, 33, 2137–2143. [Google Scholar] [CrossRef]
- Villa, E.M.; Ohlin, C.A.; Balogh, E.; Anderson, T.M.; Nyman, M.D.; Casey, W.H. Reaction Dynamics of the Decaniobate Ion [HxNb10O28](6−x)− in Water. Angew. Chem. Int. Ed. 2008, 47, 4844–4846. [Google Scholar] [CrossRef]
- Matsumoto, M.; Ozawa, Y.; Yagasaki, A.; Zhe, Y. Decatantalate—The Last Member of the Group 5 Decametalate Family. Inorg. Chem. 2013, 52, 7825–7827. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Ozawa, Y.; Isobe, K. The first vanadate hexamer capped by 4 cyclopentadienyl-rhodium or cyclopentadienyl-iridium groups. Chem. Lett. 1989, 425–428. [Google Scholar]
- Chae, H.K.; Klemperer, W.G.; Day, V.W. Organometal hydroxide route to [(C5Me5)Rh]4(V6O19). Inorg. Chem. 1989, 28, 1423–1424. [Google Scholar]
- Ma, P.T.; Chen, G.; Wang, G.; Wang, J.P. Cobalt—Sandwiched lindqvist hexaniobate dimer [Co(III)H5(Nb6O19)2]8−. Rus. J. Coord. Chem. 2011, 37, 772–775. [Google Scholar] [CrossRef]
- Antonio, M.R.; Nyman, M.; Anderson, T.M. Direct Observation of Contact Ion-Pair Formation in Aqueous Solution. Angew. Chem. Int. Ed. 2009, 48, 6136–6140. [Google Scholar] [CrossRef]
- Dale, B.W.; Buckley, J.M.; Pope, M.T. Heteropoly-niobates and -tantalates containing manganese(IV). J. Chem. Soc. A. 1969, 301–304. [Google Scholar] [CrossRef]
- Flynn, C.M., Jr.; Stucky, G.D. Sodium 6-niobo(ethylenediamine)cobaltate(III) and its chromate(III) analog. Inorg. Chem. 1969, 8, 178–180. [Google Scholar] [CrossRef]
- Bontchev, R.P.; Nyman, M. Evolution of Polyoxoniobate Cluster Anions. Angew. Chem., Int. Ed. 2006, 45, 6670–6672. [Google Scholar] [CrossRef]
- Niu, J.Y.; Ma, P.T.; Niu, H.Y.; Li, J.; Zhao, J.W.; Song, Y.; Wang, J.P. Giant Polyniobate Clusters Based on [Nb7O22]9− Units Derived from a Nb6O19 Precursor. Chem.–Eur. J. 2007, 13, 8739–8748. [Google Scholar] [CrossRef]
- Chen, G.; Ma, P.T.; Wang, J.P.; Niu, J.Y. A new organic–inorganic hybrid polyoxoniobate based on Lindqvist-type anion and nickel complex. J. Coord. Chem. 2010, 63, 3753–3763. [Google Scholar] [CrossRef]
- Hegetschweiler, K.; Finn, R.C.; Rarig, R.S.; Sander, J.; Steinhauser, S.; Worle, M.; Zubieta, J. Surface complexation of [Nb6O19]8− with NiII: solvothermal synthesis and X-ray structural characterization of two novel heterometallic Ni-Nb-polyoxometalates. J. Inorg. Chim. Acta 2002, 337, 39–47. [Google Scholar] [CrossRef]
- Dickman, M.H.; Pope, M.T. Robust, Alkali-Stable, Triscarbonyl Metal Derivatives of Hexametalate Anions, [M6O19{M‘(CO)3}n](8−n)− (M = Nb, Ta; M‘ = Mn, Re; n = 1, 2). Inorg. Chem. 2001, 40, 2582–2586. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, D.; Thouvenot, R.; Boubekeur, K.; Proust, A. Synthesis and reactivity of {Ru(p-cymene)}2+ derivatives of [Nb6O19]8−: A rational approach towards fluxional organometallic derivatives of polyoxometalates. Dalton Trans. 2007, 1334–1345. [Google Scholar] [CrossRef]
- Hill, C.L.; Prosser-McCartha, C.M. Homogeneous catalysis by transition metal oxygen anion clusters. Coord. Chem. Rev. 1995, 143, 407–455. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Tkachev, S.; Baidina, I.; Korenev, S. Speciation of Platinum(IV) in Nitric Acid Solutions. Inorg. Chem. 2013, 52, 10532–10541. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Qin, C.; Su, Z.-M.; Xing, Y.; Lang, X.-L.; Shao, K.-Z.; Lan, Y.-Q.; Wang, E.-B. Self-Assembly and Photocatalytic Properties of Polyoxoniobates: {Nb24O72}, {Nb32O96}, and {K12Nb96O288} Clusters. J. Am. Chem. Soc. 2012, 134, 14004–14010. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.; Joo, H.C.; Park, K.M.; Mal, S.S.; Kortz, U.; Keita, B.; Nadjo, V. Facile Incorporation of Platinum(IV) into Polyoxometalate Frameworks: Preparation of [H2PtIVV9O28]5− and Characterization by 195Pt NMR Spectroscopy. Angew. Chem. Int. Ed. 2008, 47, 793–796. [Google Scholar] [CrossRef]
- Kortz, U.; Lee, U.; Joo, H.C.; Park, K.M.; Mal, S.S.; Dickman, M.H.; Jameson, G.B. Platinum-Containing Polyoxometalates. Angew. Chem. Int. Ed. 2008, 47, 9383–9384. [Google Scholar] [CrossRef]
- Abramov, P.A.; Sokolov, M.N.; Virovets, A.V.; Floquet, S.; Haouas, M.; Taulelle, F.; Cadot, E.; Vicent, C.; Fedin, V. Grafting {Cp*Rh}2+ on the surface of Nb and Ta Lindqvist-type POM. Dalton Trans. 2015, 44, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, V.B.; Bombi, G.G. Electrospray mass spectrometry (ESI-MS) in the study of metal-ligand solution equilibria. Mass Spectrom. Rev. 2006, 25, 347–379. [Google Scholar] [CrossRef] [PubMed]
- Ohlin, C.A. Reaction Dynamics and Solution Chemistry of Polyoxometalates by Electrospray Ionization Mass Spectrometry. Chem.-Asian J. 2012, 7, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Abramov, P.A.; Sokolov, M.N.; Floquet, S.; Haouas, V.; Taulelle, V.; Cadot, V.; Peresypkina, E.V.; Virovets, A.V.; Vicent, C.; Kompankov, N.B.; Zhdanov, A.A.; Shuvaeva, O.V.; Fedin, V.P. Coordination-Induced Condensation of [Ta6O19]8–: Synthesis and Structure of [{(C6H6)Ru}2Ta6O19]4– and [{(C6H6)RuTa6O18}2(μ-O)]10−. Inorg. Chem. 2014, 53, 12791–12798. [Google Scholar] [CrossRef] [PubMed]
- Abramov, P.A.; Vicent, C.; Kompankov, N.B.; Gushchin, A.L.; Sokolov, M.N. Platinum polyoxoniobates. Chem. Commun. 2015, 51, 4021–4023. [Google Scholar] [CrossRef]
- Miras, H.N.; Wilson, E.F.; Cronin, L. Unravelling the complexities of inorganic and supramolecular self-assembly in solution with electrospray and cryospray mass spectrometry. Chem. Commun. 2009, 1297–1311. [Google Scholar] [CrossRef]
- Sokolov, M.N.; Adonin, S.A.; Abramov, P.A.; Mainichev, D.A.; Zakharchuk, N.F.; Fedin, V.P. Self-assembly of polyoxotungstate with tetrarhodium-core: Synthesis, structure and 183W NMR studies. Chem. Commun. 2012, 48, 6666–6668. [Google Scholar] [CrossRef]
- Ray, S.K. Synthesis of a Te dodecaniobate. J. Ind. Chem. Soc. 1976, 53, 1238–1239. [Google Scholar]
- Son, J.-H.; Wang, J.; Osterloh, F.E.; Yu, P.; Casey, W.H. A tellurium-substituted Lindqvist-type polyoxoniobate showing high H2 evolution catalyzed by tellurium nanowires via photodecomposition. Chem. Commun. 2014, 50, 836–838. [Google Scholar] [CrossRef]
- Nyman, M.; Bonhomme, F.; Alam, T.M.; Parise, J.B.; Vaughan, G.M.B. [SiNb12O40]16− and [GeNb12O40]16−: Highly Charged Keggin Ions with Sticky Surfaces. Angew. Chem., Int. Ed. 2004, 43, 2787–2792. [Google Scholar] [CrossRef]
- Anyushin, A.V.; Smolentsev, A.I.; Mainichev, D.A.; Vicent, C.; Gushchin, A.L.; Sokolov, M.N.; Fedin, V.P. Synthesis and characterization of a new Keggin anion: [BeW12O40]6−. Chem. Commun. 2014, 50, 9083. [Google Scholar] [CrossRef]
- Black, J.R.; Nyman, M.; Casey, W.H. Kinetics of 17O-exchange reactions in aqueous metal-oxo nanoclusters. Geochim. Cosmochim. Acta 2006, 70, A53–A53. [Google Scholar]
- Nelson, W.H.; Tobias, R.S. Polyanions of the Transition Metals. II. Ultracentrifugation of Alkaline Tantalum(V) Solutions; Comparison with Light Scattering. Inorg. Chem. 1964, 3, 653–658. [Google Scholar] [CrossRef]
- Spinner, B.; Kheddar, N. Nouveaux isopolyanions du tantale V. Comp. Rend. 1969, 268C, 1108–1111. [Google Scholar]
- Arana, G.; Etxebarria, N.; Fernandez, L.A.; Madariaga, J.M. Hydrolysis of Nb(V) and Ta(V) in aqueous KCl at 25 °C. Part II: Construction of a thermodynamic model for Ta(V). J. Solution Chem. 1995, 24, 611–622. [Google Scholar] [CrossRef]
- Matsumoto, M.; Ozawa, Y.; Yagasaki, A. Which is the most basic oxygen in [Ta6O19]8−?—Synthesis and structural characterization of [H2Ta6O19]6−. Inorg. Chem. Commun 2011, 14, 115–117. [Google Scholar] [CrossRef]
- Fullmer, L.B.; Molina, P.I.; Antonio, M.R.; Nyman, M. Contrasting ion-association behaviour of Ta and Nb polyoxometalates. Dalton Trans. 2014, 43, 15295–15299. [Google Scholar] [CrossRef] [PubMed]
- Deblonde, G.J.P.; Moncomble, A.; Cote, G.; Belair, S.; Chagnes, A. Experimental and computational exploration of the UV-visible properties of hexaniobate and hexatantalate ions. RSC Adv. 2015, 5, 7619–7627. [Google Scholar] [CrossRef]
- Maekawa, M.; Ozawa, Y.; Yagasaki, A. Icosaniobate: A New Member of the Isoniobate Family. Inorg. Chem. 2006, 45, 9608–9609. [Google Scholar]
- Matsumoto, M.; Ozawa, Y.; Yagasaki, A. Reversible dimerization of decaniobate. Polyhedron 2010, 29, 2196–2201. [Google Scholar] [CrossRef]
- Tsunashima, R.; Long, D.L.; Miras, H.N.; Gabb, D.; Pradeep, C.P.; Cronin, L. The Construction of High-Nuclearity Isopolyoxoniobates with Pentagonal Building Blocks: [HNb27O76]16− and [H10Nb31O93(CO3)]23−. Angew. Chem. Int. Ed. 2010, 49, 113–116. [Google Scholar] [CrossRef]
- Villa, E.M.; Ohlin, C.A.; Rustad, J.R.; Casey, W.H. Isotope-Exchange Dynamics in Isostructural Decametalates with Profound Differences in Reactivity. J. Am. Chem. Soc. 2009, 131, 16488–16492. [Google Scholar] [CrossRef]
- Villa, E.M.; Ohlin, C.A.; Casey, W.H. Oxygen-Isotope Exchange Rates for Three Isostructural Polyoxometalate Ions. J. Am. Chem. Soc. 2010, 132, 5264–5272. [Google Scholar] [CrossRef] [PubMed]
- Bannani, F.; Floquet, S.; Leclerc-Laronze, N.; Haouas, M.; Taulelle, F.; Marrot, J.; Kögerler, P.; Cadot, E. Cubic Box versus Spheroidal Capsule Built from Defect and Intact Pentagonal Units. J. Am. Chem. Soc. 2012, 134, 19342–19345. [Google Scholar] [CrossRef] [PubMed]
- Van Lokeren, L.; Cartuyvels, E.; Absillis, G.; Willema, R.; Parac-Vogt, T.N. Phosphoesterase activity of polyoxomolybdates: as a tool for obtaining insights into the reactivity of polyoxometalate clusters. Chem. Commun. 2008, 2774–2776. [Google Scholar] [CrossRef]
- Lemonnier, J.-F.; Floquet, S.; Kachmar, A.; Rohmer, M.-M.; Bénard, M.; Marrot, J.; Terazzi, E.; Piguet, C.; Cadot, E. Host–guest adaptability within oxothiomolybdenum wheels: structures, studies in solution and DFT calculations. Dalton Trans. 2007, 3043–3054. [Google Scholar] [CrossRef]
- Sakurai, N.; Kadohata, K.; Ichinose, N. Application of high-speed liquid chromatography using solvent extraction of the molybdoheteropoly yellow to the determination of microamounts of phosphorus in waste waters. Fresenius' Z. Anal. Chem. 1983, 314, 634–637. [Google Scholar] [CrossRef]
- Kirk, A.D.; Riske, W.; Lyon, D.K.; Rapko, B.; Finke, R.G. Rapid, high-resolution, reversed-phase HPLC separation of highly charged polyoxometalates using ion-interaction reagents and competing ions. Inorg. Chem. 1989, 28, 792–797. [Google Scholar] [CrossRef]
- Hettiarachichi, K.; Ha, Y.; Tran, T.; Cheung, A.P. Application of HPLC and CZE to the analysis of polyoxometalates. J. Pharm. Biomed. An. 1995, 13, 515–523. [Google Scholar] [CrossRef]
- Oliveri, A.F.; Elliott, E.W.; Carnes, M.E.; Hutchison, J.E.; Johnson, D.W. Elucidating Inorganic Nanoscale Species in Solution: Complementary and Corroborative Approaches. ChemPhysChem 2013, 14, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramov, P.A.; Sokolov, M.N.; Vicent, C. Polyoxoniobates and Polyoxotantalates as Ligands—Revisited. Inorganics 2015, 3, 160-177. https://doi.org/10.3390/inorganics3020160
Abramov PA, Sokolov MN, Vicent C. Polyoxoniobates and Polyoxotantalates as Ligands—Revisited. Inorganics. 2015; 3(2):160-177. https://doi.org/10.3390/inorganics3020160
Chicago/Turabian StyleAbramov, Pavel A., Maxim N. Sokolov, and Cristian Vicent. 2015. "Polyoxoniobates and Polyoxotantalates as Ligands—Revisited" Inorganics 3, no. 2: 160-177. https://doi.org/10.3390/inorganics3020160
APA StyleAbramov, P. A., Sokolov, M. N., & Vicent, C. (2015). Polyoxoniobates and Polyoxotantalates as Ligands—Revisited. Inorganics, 3(2), 160-177. https://doi.org/10.3390/inorganics3020160