Photo-Modulation of Single-Molecule Magnetic Dynamics of a Dysprosium Dinuclear Complex via a Diarylethene Bridge
Abstract
:1. Introduction
2. Results
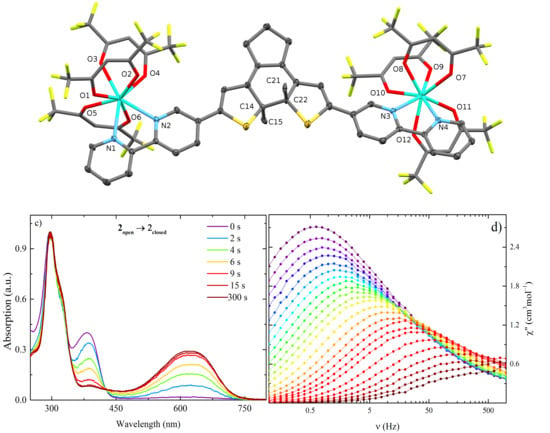
2.1. Structural Description
2.2. Optical Properties
2.3. Magnetic Properties
3. Discussion
4. Materials and Methods
4.1. Synthesis of 1open
4.2. Synthesis of 2open
4.3. Synthesis of 2close
4.4. Single-Crystal X-ray Diffraction
4.5. Physical Measurements
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Komeda, T.; Isshiki, H.; Liu, J.; Zhang, Y.-F.; Lorente, N.; Katoh, K.; Breedlove, B.K.; Yamashita, M. Observation and Electric Current Control of a Local Spin in a Single-Molecule Magnet. Nat. Commun. 2011, 2, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Yang, F.; Xin, B.; Zeng, G.; Zhou, X.; Liu, K.; Ma, D.; Li, G.; Shi, Z.; Feng, S. Reversible Switching of Slow Magnetic Relaxation in a Classic Lanthanide Metal–Organic Framework System. Chem. Commun. 2013, 49, 8244–8246. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-L.; Chen, Y.-C.; Zheng, Y.-Z.; Lin, W.-Q.; Ungur, L.; Wernsdorfer, W.; Chibotaru, L.F.; Tong, M.-L. Switching the Anisotropy Barrier of a Single-Ion Magnet by Symmetry Change from Quasi-D5h to Quasi-Oh. Chem. Sci. 2013, 4, 3310–3316. [Google Scholar] [CrossRef]
- Feng, X.; Mathonière, C.; Jeon, I.-R.; Rouzières, M.; Ozarowski, A.; Aubrey, M.L.; Gonzalez, M.I.; Clérac, R.; Long, J.R. Tristability in a Light-Actuated Single-Molecule Magnet. J. Am. Chem. Soc. 2013, 135, 15880–15884. [Google Scholar] [CrossRef] [PubMed]
- Kuch, W. Magnetic Nanostructures: Edge Atoms Do All the Work. Nat. Mater. 2003, 2, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Thirion, C.; Wernsdorfer, W.; Mailly, D. Switching of Magnetization by Nonlinear Resonance Studied in Single Nanoparticles. Nat. Mater. 2003, 2, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Gütlich, P.; Garcia, Y.; Woike, T. Photoswitchable Coordination Compounds. Coord. Chem. Rev. 2001, 219, 839–879. [Google Scholar] [CrossRef]
- Fetoh, A.; Cosquer, G.; Morimoto, M.; Irie, M.; El-Gammal, O.; El-Reash, G.A.; Breedlove, B.K.; Yamashita, M. Photo-Activation of Single Molecule Magnet Behavior in a Manganese-Based Complex. Sci. Rep. 2016, 6, 23785. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Miyasaka, H.; Yamashita, M.; Irie, M. Coordination Assemblies of [Mn4] Single-Molecule Magnets Linked by Photochromic Ligands: Photochemical Control of the Magnetic Properties. J. Am. Chem. Soc. 2009, 131, 9823–9835. [Google Scholar] [CrossRef] [PubMed]
- Shiga, T.; Miyasaka, H.; Yamashita, M.; Morimoto, M.; Irie, M. Copper(II)-terbium(III) Single-Molecule Magnets Linked by Photochromic Ligands. Dalt. Trans. 2011, 40, 2275–2282. [Google Scholar] [CrossRef] [PubMed]
- Pinkowicz, D.; Ren, M.; Zheng, L.-M.; Sato, S.; Hasegawa, M.; Morimoto, M.; Irie, M.; Breedlove, B.K.; Cosquer, G.; Katoh, K.; et al. Control of the Single-Molecule Magnet Behavior of Lanthanide-Diarylethene Photochromic Assemblies by Irradiation with Light. Chem. Eur. J. 2014, 20, 12502–12513. [Google Scholar] [CrossRef] [PubMed]
- Cosquer, G.; Morimoto, M.; Irie, M.; Fetoh, A.; Breedlove, B.K.; Yamashita, M. Photo-Control of the Magnetic Properties of Dy(III) and Ho(III) Homometal Coordination Polymers Bridged by a Diarylethene Ligand. Dalt. Trans. 2015, 44, 5996–6002. [Google Scholar] [CrossRef] [PubMed]
- Kobatake, S.; Uchida, K.; Tsuchida, E.; Irie, M. Single-Crystalline Photochromism of Diarylethenes: Reactivity—Structure Relationship. Chem. Commun. 2002, 2, 2804–2805. [Google Scholar] [CrossRef]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape Maps and Polyhedral Interconversion Paths in Transition Metal Chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Ruiz-Martínez, A.; Casanova, D.; Alvarez, S. Polyhedral Structures with an Odd Number of Vertices: Nine-Coordinate Metal Compounds. Chem. Eur. J. 2008, 14, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Guerchais, V.; Ordronneau, L.; Le Bozec, H. Recent Developments in the Field of Metal Complexes Containing Photochromic Ligands: Modulation of Linear and Nonlinear Optical Properties. Coord. Chem. Rev. 2010, 254, 2533–2545. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; Wiley-VCH: New York, NY, USA, 1993. [Google Scholar]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Ungur, L.; Sigrist, M.; Sundt, A.; Schau-magnussen, M.; Vieru, V.; Mutka, H.; Rols, S.; Weihe, H.; Waldmann, O.; et al. Modifying the Properties of 4f Single-Ion Magnets by Peripheral Ligand Functionalisation. Chem. Sci. 2014, 5, 1650–1660. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A Complex Plane Representation of Dielectric and Mechanical Relaxation Processes in Some Polymers. Polymer 1967, 8, 161–210. [Google Scholar] [CrossRef]
- Kamila, M.; Cosquer, G.; Breedlove, B.K.; Yamashita, M. Packing Structure Effects on the Slow Magnetic Relaxation Pathways of Dysprosium (III) Complexes. Bull. Chem. Soc. Jpn. 2017, 90, 595–603. [Google Scholar] [CrossRef]
- Morimoto, M.; Irie, M. Photochromic Reactions of Diarylethenes in Single Crystals with Intermolecular O–H···N Hydrogen-Bonding Networks. Chem. Eur. J. 2006, 12, 4275–4282. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.F.; Wagner, W.F.; Sands, D.E. Rare-Earth Trishexafluoroacetylacetonates and Related Compounds. J. Inorg. Nucl. Chem. 1968, 30, 1275–1289. [Google Scholar] [CrossRef]
- CrystalClear-SM, 1.4.0 SP1; Rigaku and Rigaku/MSC: The Woodlands, TX, USA, 2008.
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.; Polidori, G.; Spagna, R. SIR97: A New Tool for Crystal Structure Determination and Refinement. J. Appl. Crystallogr. 1999, 32, 115. [Google Scholar] [CrossRef]
- Sheldrick, G. A Short History of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Liang, Z.; Damjanović, M.; Kamila, M.; Cosquer, G.; Breedlove, B.K.; Enders, M.; Yamashita, M. Proton Control of the Lanthanoid Single-Ion Magnet Behavior of a Double-Decker Complex with an Indolenine-Substituted Annulene Ligand. Inorg. Chem. 2017, 56, 6512–6521. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosquer, G.; Kamila, M.; Li, Z.-Y.; Breedlove, B.K.; Yamashita, M. Photo-Modulation of Single-Molecule Magnetic Dynamics of a Dysprosium Dinuclear Complex via a Diarylethene Bridge. Inorganics 2018, 6, 9. https://doi.org/10.3390/inorganics6010009
Cosquer G, Kamila M, Li Z-Y, Breedlove BK, Yamashita M. Photo-Modulation of Single-Molecule Magnetic Dynamics of a Dysprosium Dinuclear Complex via a Diarylethene Bridge. Inorganics. 2018; 6(1):9. https://doi.org/10.3390/inorganics6010009
Chicago/Turabian StyleCosquer, Goulven, Mritunjoy Kamila, Zhao-Yang Li, Brian K. Breedlove, and Masahiro Yamashita. 2018. "Photo-Modulation of Single-Molecule Magnetic Dynamics of a Dysprosium Dinuclear Complex via a Diarylethene Bridge" Inorganics 6, no. 1: 9. https://doi.org/10.3390/inorganics6010009
APA StyleCosquer, G., Kamila, M., Li, Z. -Y., Breedlove, B. K., & Yamashita, M. (2018). Photo-Modulation of Single-Molecule Magnetic Dynamics of a Dysprosium Dinuclear Complex via a Diarylethene Bridge. Inorganics, 6(1), 9. https://doi.org/10.3390/inorganics6010009