Hydrotalcite-Type Materials Electrodeposited on Open-Cell Metallic Foams as Structured Catalysts
Abstract
:1. Introduction
2. Reactions Involved in the Electrodeposition of HT Compounds and Nitrate Concentration Effect
3. Effect of Electrosynthesis Parameters in the Preparation of Ni/Al and Rh/Mg/Al HT Materials for Structured Catalysts
3.1. Ni/Al Catalysts
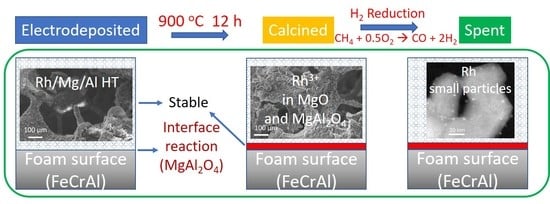
3.2. Rh/Mg/Al Catalysts
3.2.1. pH of the Electrolyte, KNO3 Supporting Electrolyte and Synthesis Conditions
3.2.2. Rh Loading, Electrolyte Concentration and Bimetallic Catalysts
3.2.3. Double compartment Flow Cell
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tronconi, E.; Groppi, G.; Visconti, C.G. Structured catalysts for non-adiabatic applications. Curr. Opin. Chem. Eng. 2014, 5, 55–67. [Google Scholar] [CrossRef]
- Montebelli, A.; Visconti, C.G.; Groppi, G.; Tronconi, E.; Cristiani, C.; Ferreira, C.; Kohlerd, S. Methods for the catalytic activation of metallic structured substrates. Catal. Sci. Technol. 2014, 4, 2846–2870. [Google Scholar] [CrossRef]
- Bianchi, E.; Heidig, T.; Visconti, C.G.; Groppi, G.; Freund, H.; Tronconi, E. An appraisal of the heat transfer properties of metallic open-cell foams for strongly exo-/endo-thermic catalytic processes in tubular reactors. Chem. Eng. J. 2012, 198–199, 512–528. [Google Scholar] [CrossRef]
- Bianchi, E.; Heidig, T.; Visconti, C.G.; Groppi, G.; Freund, H.; Tronconi, E. Heat transfer properties of metal foam supports for structured catalysts: Wall heat transfer coefficient. Catal. Today 2013, 216, 121–134. [Google Scholar] [CrossRef]
- Klumpp, M.; Inayat, A.; Schwerdtfeger, J.; Körner, C.; Singer, R.F.; Freund, H.; Schwieger, W. Periodic open cellular structures with ideal cubic cell geometry: Effect of porosity and cell orientation on pressure drop behavior. Chem. Eng. J. 2014, 242, 364–378. [Google Scholar] [CrossRef]
- Inayat, A.; Klumpp, M.; Lämmermann, M.; Freund, H.; Schwieger, W. Development of a new pressure drop correlation for open-cell foams based completely on theoretical grounds: Taking into account strut shape and geometric tortuosity. Chem. Eng. J. 2016, 287, 704–719. [Google Scholar] [CrossRef]
- Giani, L.; Cristiani, C.; Groppi, G.; Tronconi, E. Washcoating method for Pd/γ-Al2O3 deposition on metallic foams. Appl. Catal. B Environ. 2006, 62, 121–131. [Google Scholar] [CrossRef]
- Cristiani, C.; Finocchio, E.; Latorrata, S.; Visconti, C.G.; Bianchi, E.; Tronconi, E.; Groppi, G.; Pollesel, P. Activation of metallic open-cell foams via washcoat deposition of Ni/MgAl2O4 catalysts for steam reforming reaction. Catal. Today 2012, 197, 256–264. [Google Scholar] [CrossRef]
- Montebelli, A.; Visconti, C.G.; Groppi, G.; Tronconi, E.; Kohler, S.; Venvik, H.J.; Myrstad, R. Washcoating and chemical testing of a commercial Cu/ZnO/Al2O3 catalyst for the methanol synthesis over copper open-cell foams. Appl. Catal. A Gen. 2014, 481, 96–103. [Google Scholar] [CrossRef]
- Balzarotti, R.; Cristiani, C.; Francisc, L.F. Spin coating deposition on complex geometry substrates: Influence of operative parameters. Surf. Coat. Technol. 2017, 330, 1–9. [Google Scholar] [CrossRef]
- Bortolozzi, J.P.; Weiss, T.; Gutierrez, L.B.; Ulla, M.A. Comparison of Ni and Ni–Ce/Al2O3 catalysts in granulated and structured forms: Their possible use in the oxidative dehydrogenation of ethane reaction. Chem. Eng. J. 2014, 246, 343–352. [Google Scholar] [CrossRef]
- Banús, E.D.; Ulla, M.A.; Miró, E.E.; Milt, V.G. Co,Ba,K/ZrO2 coated onto metallic foam (AISI 314) as a structured catalyst for soot combustion: Catalytic activity and stability. Appl. Catal. A Gen. 2011, 393, 9–16. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Yu, H.; Peng, F.; Wang, H. Metal-Foam-Supported Pd/Al2O3 Catalysts for Catalytic Combustion of Methane: Effect of Interaction between Support and Catalyst. Int. J. Chem. React. Eng. 2015, 13, 83–93. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Li, H.; Cai, S.; Hu, H.; Fang, C.; Shi, L.; Zhang, D. Rational design and in situ fabrication of MnO2@NiCo2O4 nanowire arrays on Ni foam as high-performance monolith de-NOx catalysts. J. Mater. Chem. A 2015, 3, 11543–11553. [Google Scholar] [CrossRef]
- Fang, C.; Shi, L.; Hu, H.; Zhang, J.; Zhang, D. Rational design of 3D hierarchical foam-like Fe2O3@CuOx monolith catalysts for selective catalytic reduction of NO with NH3. RSC Adv. 2015, 5, 11013–11022. [Google Scholar] [CrossRef]
- Kryca, J.; Jodłowski, P.J.; Iwaniszyn, M.; Gil, B.; Sitarz, M.; Kołodziej, A.; Łojewska, T.; Łojewska, J. Cu SSZ-13 zeolite catalyst on metallic foam support for SCR of NOx with ammonia: Catalyst layering and characterisation of active sites. Catal. Today 2016, 268, 142–149. [Google Scholar] [CrossRef]
- Liang, Z.; Gao, P.; Tang, Z.; Lv, M.; Sun, Y. Three dimensional porous Cu–Zn/Al foam monolithic catalyst for CO2 hydrogenation to methanol in microreactor. J. CO2 Utiliz. 2017, 21, 191–199. [Google Scholar] [CrossRef]
- Freya, M.; Romero, T.; Roger, A.-C.; Edouard, D. Open cell foam catalysts for CO2 methanation: Presentation of coating procedures and in situ exothermicity reaction study by infrared thermography. Catal. Today 2016, 273, 83–90. [Google Scholar] [CrossRef]
- Méndez, F.J.; Sanz, O.; Montes, M.; Guerra, J.; Olivera-Fuentes, C.; Curbelo, S.; Brito, J.L. Selective hydrogenation of 1,3-butadiene in the presence of 1-butene under liquid phase conditions using structured catalysts. Catal. Today 2017, 289, 151–161. [Google Scholar] [CrossRef]
- Chai, R.; Li, Y.; Zhang, Q.; Fan, S.; Zhang, Z.; Chen, P.; Zhao, G.; Liu, Y.; Lu, Y. Foam-Structured NiO–MgO–Al2O3 Nanocomposites Derived from NiMgAl Layered Double Hydroxides In Situ Grown onto Nickel Foam: A Promising Catalyst for High-Throughput Catalytic Oxymethane Reforming. ChemCatChem 2017, 9, 268–272. [Google Scholar] [CrossRef]
- Cao, C.; Xing, L.; Yang, Y.; Tian, Y.; Ding, T.; Zhang, J.; Hu, T.; Zheng, L.; Li, X. The monolithic transition metal oxide crossed nanosheets used for diesel soot combustion under gravitational contact mode. Appl. Surf. Sci. 2017, 406, 245–253. [Google Scholar] [CrossRef]
- Therese, G.H.A.; Kamath, P.V. Electrochemical Synthesis of Metal Oxides and Hydroxides. Chem. Mater. 2000, 12, 1195–1204. [Google Scholar] [CrossRef]
- Cattarin, S.; Musiani, M. Electrosynthesis of nanocomposite materials for electrocatalysis. Electrochim. Acta 2007, 52, 2796–2805. [Google Scholar] [CrossRef]
- Bicelli, L.P.; Bozzini, B.; Mele, C.; D’Urzo, L. A Review of Nanostructural Aspects of Metal Electrodeposition. Int. J. Electrochem. Sci. 2008, 3, 356–408. [Google Scholar]
- Chandrasekar, M.S.; Pushpavanam, M. Pulse and pulse reverse plating—Conceptual, advantages and applications. Electrochim. Acta 2008, 53, 3313–3322. [Google Scholar] [CrossRef]
- Gurrappa, I.; Binder, L. Electrodeposition of nanostructured coatings and their characterization—A Review. Sci. Technol. Adv. Mater. 2008, 9, 043001. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-S.; Jang, H.S.; McShane, C.M.; Read, C.G.; Seabold, J.A. Electrochemical Synthesis of Inorganic Polycrystalline Electrodes with Controlled Architectures. MRS Bull. 2010, 35, 753–760. [Google Scholar] [CrossRef]
- Wu, X.-J.; Zhu, F.; Mu, C.; Liang, Y.; Xu, L.; Chen, Q.; Chen, R.; Xu, D. Electrochemical synthesis and applications of oriented and hierarchically quasi-1D semiconducting nanostructures. Coord. Chem. Rev. 2010, 254, 1135–1150. [Google Scholar] [CrossRef]
- Mohanty, U.S. (Ed.) Electrodeposition: Properties, Processes and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2012. [Google Scholar]
- Kang, D.; Kim, T.W.; Kubota, S.R.; Cardiel, A.C.; Cha, H.G.; Choi, K.-S. Electrochemical Synthesis of Photoelectrodes and Catalysts for Use in Solar Water Splitting. Chem. Rev. 2015, 115, 12839–12887. [Google Scholar] [CrossRef] [PubMed]
- Ustarroz, J.; Hubin, A.; Terryn, H. Supported Nanoparticle Synthesis by Electrochemical Deposition in Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer International Publishing: Basel, Switzerland, 2016; pp. 603–631. [Google Scholar]
- Roger, I.; Symes, M.D. First row transition metal catalysts for solar-driven water oxidation produced by electrodeposition. J. Mater. Chem. A 2016, 4, 6724–6741. [Google Scholar] [CrossRef] [Green Version]
- Ng, P.K.; Schneider, E.W. Distribution of Nickel Hydroxide in Sintered Nickel Plaques Measured by Radiotracer Method during Electroimpregnation. J. Electrochem. Soc. 1986, 133, 17–21. [Google Scholar] [CrossRef]
- Ho, K.C. Electrochemical Precipitation of Nickel Hydroxide. J. Electrochem. Soc. 1987, 134, 52C–55C. [Google Scholar] [CrossRef]
- Indira, L.; Kamath, P.V. Electrogeneration of base by cathodic reduction of anions: Novel one-step route to unary and layered double hydroxides (LDHs). J. Mater. Chem. 1994, 4, 1487–1490. [Google Scholar] [CrossRef]
- Dixit, M.; Kamath, P.V. Electrosynthesis and stabilization of α-cobalt hydroxide in the presence of trivalent cations. J. Power Sources 1995, 56, 97–100. [Google Scholar] [CrossRef]
- Tonelli, D.; Scavetta, E.; Giorgetti, M. Layered-double-hydroxide-modified electrodes: Electroanalytical applications. Anal. Bioanal. Chem. 2013, 405, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Zhang, R.; Li, Z.; Wei, M.; Evans, D.G.; Duan, X. Layered double hydroxides toward electrochemical energy storage and conversion: Design, synthesis and applications. Chem. Commun. 2015, 51, 15880–15893. [Google Scholar] [CrossRef] [PubMed]
- Vlamidis, Y.; Scavetta, E.; Giorgetti, M.; Sangiorgi, N.; Tonelli, D. Electrochemically synthesized cobalt redox active layered double hydroxides for supercapacitors development. Appl. Clay Sci. 2017, 143, 151–158. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Xiang, X.; Yan, D.; Li, F. Engineering of ZnCo-layered double hydroxide nanowalls toward high-efficiency electrochemical water oxidation. J. Mater. Chem. A 2014, 2, 13250–13258. [Google Scholar] [CrossRef]
- Burke, M.S.; Kast, M.G.; Trotochaud, L.; Smith, A.M.; Boettcher, S.W. Cobalt−Iron (Oxy)hydroxide Oxygen Evolution Electrocatalysts: The Role of Structure and Composition on Activity, Stability, and Mechanism. J. Am. Chem. Soc. 2015, 137, 3638–3648. [Google Scholar] [CrossRef] [PubMed]
- Vlamidis, Y.; Scavetta, E.; Gazzano, M.; Tonelli, D. Iron vs Aluminum Based Layered Double Hydroxides as Water Splitting Catalysts. Electrochim. Acta 2016, 188, 653–660. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, N.; Zhang, G.; Xu, M.; Lu, W.; Zhou, L.; Huan, H. Design of Hierarchical Ni-Co@Ni-Co Layered Double Hydroxide Core–Shell Structured Nanotube Array for High-Performance Flexible All-Solid-State Battery-Type Supercapacitors. Adv. Funct. Mater. 2017, 27, 1605307. [Google Scholar] [CrossRef]
- Jagadale, A.D.; Guan, G.; Li, X.; Du, X.; Ma, X.; Hao, X.; Abudula, A. Binder-Free Electrodes of CoAl Layered Double Hydroxide on Carbon Fibers for All-Solid-State Flexible Yarn Supercapacitors. Energy Technol. 2016, 4, 997–1004. [Google Scholar] [CrossRef]
- Li, H.; Musharavati, F.; Zalenezhad, E.; Chen, X.; Hui, K.N.; Hui, K.S. Electrodeposited Ni–Co layered double hydroxides on titanium carbide as a binder-free electrode for supercapacitors. Electrochim. Acta 2018, 261, 178–187. [Google Scholar] [CrossRef]
- Liu, Y.; Teng, X.; Mi, Y.; Chen, Z. A new architecture design of Ni–Co LDH-based Pseudocapacitors. J. Mater. Chem. A 2017, 5, 24407–24415. [Google Scholar] [CrossRef]
- Bo, X.; Li, Y.; Hocking, R.K.; Zhao, C. NiFeCr Hydroxide Holey Nanosheet as Advanced Electrocatalyst for Water Oxidation. ACS Appl. Mater. Interfaces 2017, 9, 41239–41245. [Google Scholar] [CrossRef] [PubMed]
- Basile, F.; Benito, P.; Del Gallo, P.; Fornasari, G.; Gary, D.; Rosetti, V.; Scavetta, E.; Tonelli, D.; Vaccari, A. Highly conductive Ni steam reforming catalysts prepared by electrodeposition. Chem. Commun. 2008, 2917–2919, 2917–2919. [Google Scholar] [CrossRef] [PubMed]
- Verlato, E.; Barison, S.; Cimino, S.; Dergal, F.; Lisi, L.; Mancino, G.; Musiani, M.; Vazquez-Gómez, L. Catalytic partial oxidation of methane over nanosized Rh supported on Fecralloy foams. Int. J. Hydrogen Energy 2014, 39, 11473–11485. [Google Scholar] [CrossRef]
- Cimino, S.; Gambirasi, A.; Lisi, L.; Mancino, G.; Musiani, M.; Vázquez-Gómez, L.; Verlato, E. Catalytic combustion of methanol on Pt–Fecralloy foams prepared by electrodeposition. Chem. Eng. J. 2016, 285, 276–285. [Google Scholar] [CrossRef]
- Verlato, E.; Barison, S.; Cimino, S.; Lisi, L.; Mancino, G.; Musiani, M.; Paolucci, F. Electrochemical preparation of nanostructured CeO2-Pt catalysts on Fe-Cr-Al alloy foams for the low-temperature combustion of methanol. Chem. Eng. J. 2017, 317, 551–560. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Ho, P.H.; Monti, M.; Scavetta, E.; Tonelli, D.; Bernardi, E.; Nobili, L.; Fornasari, G.; Vaccari, A.; Benito, P. Reactions involved in the electrodeposition of hydrotalcite-type compounds on FeCrAlloy foams and plates. Electrochim. Acta 2016, 222, 1335–1344. [Google Scholar] [CrossRef]
- Milhano, C.; Pletcher, D. The Electrochemistry and Electrochemical Technology of Nitrate. In Modern Aspects of Electrochemistry, No. 45; White, R.E., Ed.; Springer: New York, NY, USA, 2009; pp. 1–61. [Google Scholar]
- Ho, P.H.; Scavetta, E.; Ospitali, F.; Tonelli, D.; Fornasari, G.; Vaccari, A.; Benito, P. Effect of metal nitrate concentration on the electrodeposition of hydrotalcite-like compounds on open-cell foams. Appl. Clay Sci. 2018, 151, 109–117. [Google Scholar] [CrossRef]
- Basile, F.; Benito, P.; Fornasari, G.; Vaccari, A. Hydrotalcite-type precursors of active catalysts for hydrogen production. Appl. Clay Sci. 2010, 48, 250–259. [Google Scholar] [CrossRef]
- Monti, M.; Benito, P.; Basile, F.; Fornasari, G.; Gazzano, M.; Scavetta, E.; Tonelli, D.; Vaccari, A. Electrosynthesis of Ni/Al and Mg/Al Layered Double Hydroxides on Pt and FeCrAlloy supports: Study and control of the pH near the electrode surface. Electrochim. Acta 2013, 108, 596–604. [Google Scholar] [CrossRef]
- Benito, P.; Monti, M.; Bersani, I.; Basile, F.; Fornasari, G.; Scavetta, E.; Tonelli, D.; Vaccari, A. Coating of FeCrAlloy foam with Rh catalysts: Optimization of electrosynthesis parameters and catalyst composition. Catal. Today 2012, 197, 162–169. [Google Scholar] [CrossRef]
- Benito, P.; Monti, M.; De Nolf, W.; Nuyts, G.; Janssen, G.; Fornasari, G.; Scavetta, E.; Basile, F.; Janssens, K.; Ospitali, F.; et al. Improvement in the coating homogeneity in electrosynthesized Rh structured catalysts for the partial oxidation of methane. Catal. Today 2015, 246, 154–164. [Google Scholar] [CrossRef]
- Ho, P.H.; de Nolf, W.; Ospitali, F.; Gondolini, A.; Fornasari, G.; Scavetta, E.; Tonelli, D.; Vaccari, A.; Benito, P. Coprecipitated-like hydrotalcite-derived coatings on open-cell metallic foams by electrodeposition: Rh nanoparticles on oxide layers stable under harsh reaction conditions. Appl. Catal. A Gen. 2018, 560, 12–20. [Google Scholar] [CrossRef]
- Basile, F.; Benito, P.; Fornasari, G.; Monti, M.; Scavetta, E.; Tonelli, D.; Vaccari, A. Novel Rh-based structured catalysts for the catalytic partial oxidation of methane. Catal. Today 2010, 157, 183–190. [Google Scholar] [CrossRef]
- Basile, F.; Benito, P.; Fornasari, G.; Rosetti, V.; Scavetta, E.; Tonelli, D.; Vaccari, A. Electrochemical synthesis of novel structured catalysts for H2 production. Appl. Catal. B Environ. 2009, 91, 563–572. [Google Scholar] [CrossRef]
- Benito, P.; Nuyts, G.; Monti, M.; De Nolf, W.; Fornasari, G.; Janssens, K.; Scavetta, E.; Vaccari, A. Stable Rh particles in hydrotalcite-derived catalysts coated on FeCrAlloy foams by electrosynthesis. Appl. Catal. B Environ. 2015, 179, 321–332. [Google Scholar] [CrossRef]
- Benito, P.; de Nolf, W.; Nuyts, G.; Monti, M.; Fornasari, G.; Basile, F.; Janssens, K.; Ospitali, F.; Scavetta, E.; Tonelli, D.; et al. Role of Coating-Metallic Support Interaction in the Properties of Electrosynthesized Rh-Based Structured Catalysts. ACS Catal. 2014, 4, 3779–3790. [Google Scholar] [CrossRef]
- Scavetta, E.; Mignani, A.; Prandstraller, D.; Tonelli, D. Electrosynthesis of Thin Films of Ni, Al Hydrotalcite Like Compounds. Chem. Mater. 2007, 19, 4523–4529. [Google Scholar] [CrossRef]
- Basile, F.; Benito, P.; Bugani, S.; De Nolf, W.; Fornasari, G.; Janssens, K.; Morselli, L.; Scavetta, E.; Tonelli, D.; Vaccari, A. Combined Use of Synchrotron-Radiation-Based Imaging Techniques for the Characterization of Structured Catalysts. Adv. Funct. Mater. 2010, 20, 4117–4126. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, P.H.; Scavetta, E.; Tonelli, D.; Fornasari, G.; Vaccari, A.; Benito, P. Hydrotalcite-Type Materials Electrodeposited on Open-Cell Metallic Foams as Structured Catalysts. Inorganics 2018, 6, 74. https://doi.org/10.3390/inorganics6030074
Ho PH, Scavetta E, Tonelli D, Fornasari G, Vaccari A, Benito P. Hydrotalcite-Type Materials Electrodeposited on Open-Cell Metallic Foams as Structured Catalysts. Inorganics. 2018; 6(3):74. https://doi.org/10.3390/inorganics6030074
Chicago/Turabian StyleHo, Phuoc Hoang, Erika Scavetta, Domenica Tonelli, Giuseppe Fornasari, Angelo Vaccari, and Patricia Benito. 2018. "Hydrotalcite-Type Materials Electrodeposited on Open-Cell Metallic Foams as Structured Catalysts" Inorganics 6, no. 3: 74. https://doi.org/10.3390/inorganics6030074
APA StyleHo, P. H., Scavetta, E., Tonelli, D., Fornasari, G., Vaccari, A., & Benito, P. (2018). Hydrotalcite-Type Materials Electrodeposited on Open-Cell Metallic Foams as Structured Catalysts. Inorganics, 6(3), 74. https://doi.org/10.3390/inorganics6030074