Botanically Templated Monolithic Macrostructured Zinc Oxide Materials for Photocatalysis
Abstract
:1. Introduction
2. Results and Discussion
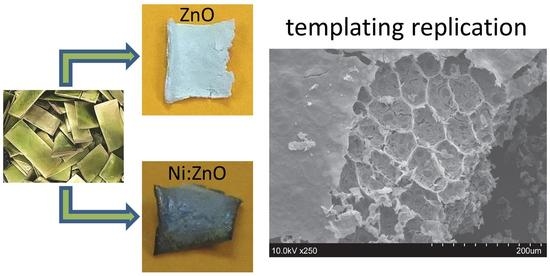
2.1. ZZ Leaf Template Characterization
2.2. Templated ZnO Characterization
2.3. Photocatalytic Dye Oxidation Studies
3. Materials and Methods
3.1. Leaf Template Preparation
3.2. Precursor Infiltration into ZZ Leaf Templates and Conversion to Zinc Oxide
3.3. Material Characterization
3.4. Photocatalytic Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Witteveen, H.J.; Farnau, E.F. Colors Developed by Cobalt Oxides. J. Ind. Eng. Chem. 1921, 13, 1061–1066. [Google Scholar] [CrossRef]
- Eastaugh, N.; Walsh, V.; Chaplin, T.; Siddall, R. Pigment Compendium: A Dictionary and Optical Microscopy of Historical Pigments; Routledge: New York, NY, USA, 2008. [Google Scholar]
- Djerdj, I.; Jaglicic, Z.; Arcon, D.; Niederberger, M. Co-Doped ZnO nanoparticles: Minireview. Nanoscale 2010, 2, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Gaudon, M.; Toulemonde, O.; Demourgues, A. Green coloration of Co-doped ZnO explained from structural refinement and bond considerations. Inorg. Chem. 2007, 46, 10996–11002. [Google Scholar] [CrossRef] [PubMed]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Rasouli, S.; Moeen, S.J. Combustion synthesis of Co-doped zinc oxide nanoparticles using mixture of citric acid–glycine fuels. J. Alloy. Compd. 2011, 509, 1915–1919. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Films 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Šutka, A.; Käämbre, T.; Pärna, R.; Juhnevica, I.; Maiorov, M.; Joost, U.; Kisand, V. Co doped ZnO nanowires as visible light photocatalysts. Solid State Sci. 2016, 56, 54–62. [Google Scholar] [CrossRef]
- Türkyılmaz, Ş.Ş.; Güy, N.; Özacar, M. Photocatalytic efficiencies of Ni, Mn, Fe and Ag doped ZnO nanostructures synthesized by hydrothermal method: The synergistic/antagonistic effect between ZnO and metals. J. Photochem. Photobiol. A 2017, 341, 39–50. [Google Scholar] [CrossRef]
- Vignesh, K.; Rajarajan, M.; Suganthi, A. Visible light assisted photocatalytic performance of Ni and Th co-doped ZnO nanoparticles for the degradation of methylene blue dye. Ind. Eng. Chem. 2014, 20, 3826–3833. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Yan, X.; Yang, Y.; Lei, Y.; Zhou, J.; Huang, Y.; Gu, Y.; Zhang, Y. Structure and photocatalytic activity of Ni-doped ZnO nanorods. Mater. Res. Bull. 2011, 46, 1207–1210. [Google Scholar] [CrossRef]
- Achouri, F.; Corbel, S.; Balan, L.; Mozet, K.; Girot, E.; Medjahdi, G.; Said, M.B.; Ghrabi, A.; Schneider, R. Porous Mn-doped ZnO nanoparticles for enhanced solar and visible light photocatalysis. Mater. Des. 2016, 101, 309–316. [Google Scholar] [CrossRef]
- Kuriakose, S.; Satpati, B.; Mohapatra, S. Enhanced photocatalytic activity of Co doped ZnO nanodisks and nanorods prepared by a facile wet chemical method. Phys. Chem. Chem. Phys. 2014, 16, 12741–12749. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhang, J.; Xiao, C.; Tan, X. Photocatalytic decolorization of methylene blue over Zn1−xCoxO under visible light irradiation. Mater. Sci. Eng. B 2007, 142, 121–125. [Google Scholar] [CrossRef]
- Emsley, J. The Elements, 2nd ed.; Oxford University Press: New York, NY, USA, 1991. [Google Scholar]
- Di Mauro, A.; Fragalà, M.E.; Privitera, V.; Impellizzeri, G. ZnO for application in photocatalysis: From thin films to nanostructures. Mater. Sci. Semicond. Process. 2017, 69, 44–51. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, C.; Ma, J.-Z. Morphology control of ZnO microstructures by varying hexamethylenetetramine and trisodium citrate concentration and their photocatalytic activity. Mater. Des. 2016, 101, 7–15. [Google Scholar] [CrossRef]
- Studart, A.R.; Gonzenbach, U.T.; Tervoort, E.; Gauckler, L.J. Processing Routes to Macroporous Ceramics: A Review. J. Am. Ceram. Soc. 2006, 89, 1771–1789. [Google Scholar] [CrossRef] [Green Version]
- Holland, B.T.; Blanford, C.F.; Do, T.; Stein, A. Synthesis of Highly Ordered, Three-Dimensional, Macroporous Structures of Amorphous or Crystalline Inorganic Oxides, Phosphates, and Hybrid Composites. Chem. Mater. 1999, 11, 795–805. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, W.; Su, H.; Fan, T.; Zhu, S.; Liu, Q.; Zhang, D. Morphology genetic materials templated from natural species. Adv. Mater. 2015, 27, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fan, T.; Zhang, D. Hydrothermal synthesis of ZnO hollow spheres using spherobacterium as biotemplates. Microporous Mesoporous Mater. 2007, 100, 322–327. [Google Scholar] [CrossRef]
- Nam, Y.S.; Magyar, A.P.; Lee, D.; Kim, J.W.; Yun, D.S.; Park, H.; Pollom, T.S., Jr.; Weitz, D.A.; Belcher, A.M. Biologically templated photocatalytic nanostructures for sustained light-driven water oxidation. Nat. Nanotechnol. 2010, 5, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Culverwell, E.; Wimbush, S.C.; Hall, S.R. Biotemplated synthesis of an ordered macroporous superconductor with high critical current density using a cuttlebone template. Chem. Commu. 2008, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhou, H.; Lou, S.; Ding, J.; Zhang, D.; Zhu, H.; Fan, T. Butterfly wing architecture assisted CdS/Au/TiO2 Z-scheme type photocatalytic water splitting. Int. J. Hydrogen Energy 2013, 38, 8244–8253. [Google Scholar] [CrossRef]
- Lu, Y.; Fong, E. Botanic chemistry enabled synthesis of 3D hollow metal oxides/carbon hybrids for ultra-high performance metal-ion batteries. Mater. Today Energy 2017, 4, 89–96. [Google Scholar] [CrossRef]
- Shchipunov, Y.; Postnova, I. Cellulose Mineralization as a Route for Novel Functional Materials. Adv. Funct. Mater. 2018, 28, 1705042. [Google Scholar] [CrossRef]
- Pushpavanam, K.; Santra, S.; Rege, K. Biotemplating plasmonic nanoparticles using intact microfluidic vasculature of leaves. Langmuir 2014, 30, 14095–14103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Zheng, X.; Wang, X.; Xu, Y.; Tang, H.; Kang, F.; Yang, Q.-H.; Luo, J. Biomass Organs Control the Porosity of Their Pyrolyzed Carbon. Adv. Funct. Mater. 2017, 27, 1604687. [Google Scholar] [CrossRef]
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef] [Green Version]
- Greil, P. Templating Approaches Using Natural Cellular Plant Tissue. Mrs. Bull. 2010, 35, 145–149. [Google Scholar] [CrossRef]
- Li, X.F.; Fan, T.X.; Zhou, H.; Chow, S.K.; Zhang, W.; Zhang, D.; Guo, Q.X.; Ogawa, H. Enhanced Light-Harvesting and Photocatalytic Properties in Morph-TiO2 from Green-Leaf Biotemplates. Adv. Funct. Mater. 2009, 19, 45–56. [Google Scholar] [CrossRef]
- Li, X.F.; Jiang, J.J.; Wang, Y.; Nie, X.; Qu, F.Y. Preparation of multilevel macroporous materials using natural plants as templates. J. Sol-Gel Sci. Technol. 2010, 56, 75–81. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Fan, T.; Osterloh, F.E.; Ding, J.; Sabio, E.M.; Zhang, D.; Guo, Q. Artificial inorganic leafs for efficient photochemical hydrogen production inspired by natural photosynthesis. Adv. Mater. 2010, 22, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.B.; Nelson, A.M.; Gillan, E.G. Titania and Silica Materials Derived from Chemically Dehydrated Porous Botanical Templates. Chem. Mater. 2012, 24, 4301–4310. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database; NIST Standard Reference Database Number 20; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000. Available online: http://srdata.nist.gov/xps/ (accessed on 30 August 2018).
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Hitchcock, P.B.; Seddon, K.R.; Welton, T. Hydrogen-bond acceptor abilities of tetrachlorometalate(II) complexes in ionic liquids. J. Chem. Soc. Dalton Trans. 1993, 2639–2643. [Google Scholar] [CrossRef]
- Cotton, F.A.; Goodgame, D.M.L.; Goodgame, M. The Electronic Structures of Tetrahedral Cobalt(II) Complexes. J. Am. Chem. Soc. 1961, 83, 4690–4699. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas Adsorption Characterization of Ordered Organic–Inorganic Nanocomposite Materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Coleman, N.; Perera, S.; Gillan, E.G. Rapid solid-state metathesis route to transition-metal doped titanias. J. Solid State Chem. 2015, 232, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Conway, K.; Kiernan, J.A. Chemical dehydration of specimens with 2,2-dimethoxypropane (DMP) for paraffin processing of animal tissues: Practical and economic advantages over dehydration in ethanol. Biotech. Histochem. 1999, 74, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.L.; Jacks, T.J. Rapid chemical dehydration of samples for electron microscopic examinations. J. Histochem. Cytochem. 1975, 23, 107–110. [Google Scholar] [CrossRef] [PubMed]









| Templated Product | ZnO Product Color | Product Yield (mmol/g) 1 | XRD Crystallite Size (nm) 2 | Zn:M Relative Atomic Ratio (EDS) | DRS Band Gap (eV) 2 |
|---|---|---|---|---|---|
| ZZ-ZnO | light beige | 7.3 | 23.0 | n/a | 3.19 |
| ZZ-Co(1%):ZnO | light green | 6.0 | 22.2 | 1:0.033 | 3.13 |
| ZZ-Co(5%):ZnO | green | 6.1 | 21.3 | 1:0.086 | 2.90 |
| ZZ-Co(10%):ZnO | dark green | 6.1 | 20.5 | 1:0.144 | 2.65 |
| ZZ-Ni(1%):ZnO | light yellow | 6.0 | 21.9 | 1:0.030 | 3.17 |
| ZZ-Ni(5%):ZnO | Yellow–gray | 6.2 | 24.6 | 1:0.074 | 3.16 |
| ZZ-Ni(10%):ZnO | dark gray | 6.0 | 25.7 | 1:0.144 | 3.15 |
| Templated Product | Zn 2p3/2 (eV) | M 2p3/2 (eV) | O1s (eV) 1 | M/Zn Atomic Ratio |
|---|---|---|---|---|
| ZZ-ZnO | 1021.5 | n/a | 530.2, 531.5 | n/a |
| ZZ-Co(1%):ZnO | 1021.5 | 781.3 | 531.4, 530.3 | 0.036 |
| ZZ-Co(5%):ZnO | 1021.4 | 780.5 | 531.2, 530.1 | 0.080 |
| ZZ-Co(10%):ZnO | 1021.3 | 779.9 | 530.9 | 0.124 |
| ZZ-Ni(1%):ZnO | 1021.7 | 855.2 | 530.8, 532.1 | 0.037 |
| ZZ-Ni(5%):ZnO | 1021.4 | 854.8 | 530.9, 531.7 | 0.167 |
| ZZ-Ni(10%):ZnO | 1021.5 | 854.5 | 531.0, 528.7 | 0.465 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Black, N.M.; Ciota, D.S.; Gillan, E.G. Botanically Templated Monolithic Macrostructured Zinc Oxide Materials for Photocatalysis. Inorganics 2018, 6, 103. https://doi.org/10.3390/inorganics6040103
Black NM, Ciota DS, Gillan EG. Botanically Templated Monolithic Macrostructured Zinc Oxide Materials for Photocatalysis. Inorganics. 2018; 6(4):103. https://doi.org/10.3390/inorganics6040103
Chicago/Turabian StyleBlack, Nathan M., David S. Ciota, and Edward G. Gillan. 2018. "Botanically Templated Monolithic Macrostructured Zinc Oxide Materials for Photocatalysis" Inorganics 6, no. 4: 103. https://doi.org/10.3390/inorganics6040103
APA StyleBlack, N. M., Ciota, D. S., & Gillan, E. G. (2018). Botanically Templated Monolithic Macrostructured Zinc Oxide Materials for Photocatalysis. Inorganics, 6(4), 103. https://doi.org/10.3390/inorganics6040103