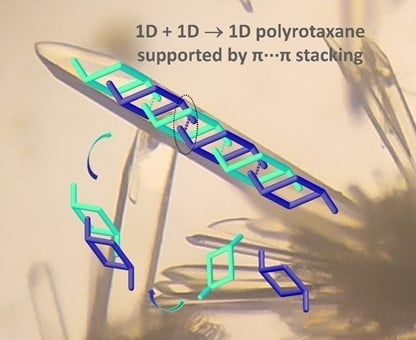
A Zn(II) Metallocycle as Platform to Assemble a 1D + 1D → 1D Polyrotaxane via π···π Stacking of an Ancillary Ligand
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis
3.1.1. Synthesis of {[Zn2L2(MeOH)2]·2CHCl3} (1)
3.1.2. Synthesis of {[Cu2L2(MeOH)2]·2CH2Cl2} (2)
3.1.3. Synthesis of {[Zn2L2(EtOH)2]·2CHCl3} (3)
3.1.4. Synthesis of [Zn2L2(iPrOH)2] (4)
3.1.5. Synthesis of {[Zn2L2](py)2·2CHCl3} (5)
3.2. Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bruns, C.J.; Stoddart, J.F. The Nature of the Mechanical Bond: From Molecules to Machines; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 9781119044123. [Google Scholar]
- Fujita, M.; Ibukuro, F.; Hagihara, H.; Ogura, K. Quantitative self-assembly of a [2]catenane from two preformed molecular rings. Nature 1994, 367, 720–723. [Google Scholar] [CrossRef]
- Fujita, M.; Fujita, N.; Ogura, K.; Yamaguchi, K. Spontaneous assembly of ten components into two interlocked, identical coordination cages. Nature 1999, 400, 52–55. [Google Scholar] [CrossRef]
- Frank, M.; Johnstone, M.D.; Clever, G.H. Interpenetrated Cage Structures. Chem. Eur. J. 2016, 22, 14104–14125. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Ferrando-Soria, J.; Pineda, E.M.; Tuna, F.; Vitorica-Yrezabal, I.J.; Knappke, C.; Ujma, J.; Muryn, C.A.; Timco, G.A.; Barran, P.E.; et al. Making hybrid [n]-rotaxanes as supramolecular arrays of molecular electron spin qubits. Nat. Commun. 2016, 7, 10240. [Google Scholar] [CrossRef]
- Lee, C.F.; Leigh, D.A.; Pritchard, R.G.; Schultz, D.; Teat, S.J.; Timco, G.A.; Winpenny, R.E.P. Hybrid organic-inorganic rotaxanes and molecular shuttles. Nature 2009, 458, 314–318. [Google Scholar] [CrossRef]
- Fernandez, A.; Moreno Pineda, E.; Ferrando-Soria, J.; McInnes, E.J.L.; Timco, G.A.; Winpenny, R.E.P. A hybrid organic–inorganic molecular daisy chain. Chem. Commun. 2015, 51, 11126–11129. [Google Scholar] [CrossRef]
- Jiang, L.; Ju, P.; Meng, X.R.; Kuang, X.J.; Lu, T.B. Constructions of two polycatenanes and one polypseudo-rotaxane by discrete tetrahedral cages and stool-like building units. Sci. Rep. 2012, 2, 668. [Google Scholar] [CrossRef]
- Truccolo, G.; Tessari, Z.; Tessarolo, J.; Quici, S.; Armelao, L.; Rancan, M. A Cu(II) metallocycle for the reversible self-assembly of coordination-driven polyrotaxane-like architectures. Dalton Trans. 2018, 47, 12079–12084. [Google Scholar] [CrossRef]
- Ju, H.; Clegg, J.K.; Park, K.M.; Lindoy, L.F.; Lee, S.S. Formation of a Dicopper Platform Based Polyrotaxane Whose “String” and “Bead” Are Constructed from the Same Components. J. Am. Chem. Soc. 2015, 137, 9535–9538. [Google Scholar] [CrossRef]
- Whitehead, G.F.S.; Cross, B.; Carthy, L.; Milway, V.A.; Rath, H.; Fernandez, A.; Heath, S.L.; Muryn, C.A.; Pritchard, R.G.; Teat, S.J.; et al. Rings and threads as linkers in metal-organic frameworks and poly-rotaxanes. Chem. Commun. 2013, 49, 7195–7197. [Google Scholar] [CrossRef]
- Clegg, J.K.; Li, F.; Jolliffe, K.A.; Lindoy, L.F.; Meehan, G.V.; Parsons, S.; Tasker, P.A.; White, F.J. Hierarchical assembly of discrete copper(II) metallo-structures from pre-assembled dinuclear (bis-β-diketonato) metallocycles and flexible difunctional co-ligands. Dalton Trans. 2013, 42, 14315–14323. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.K.; Iremonger, S.S.; Hayter, M.J.; Southon, P.D.; Macquart, R.B.; Duriska, M.B.; Jensen, P.; Turner, P.; Jolliffe, K.A.; Kepert, C.J.; et al. Hierarchical Self-Assembly of a Chiral Metal-Organic Framework Displaying Pronounced Porosity. Angew. Chemie Int. Ed. 2010, 49, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.K.; Bray, D.J.; Gloe, K.; Gloe, K.; Jolliffe, K.A.; Lawrance, G.A.; Lindoy, L.F.; Meehan, G.V.; Wenzel, M. Synthetic, structural, electrochemical and solvent extraction studies of neutral trinuclear Co(II), Ni(II), Cu(II) and Zn(II) metallocycles and tetrahedral tetranuclear Fe(III) species incorporating 1,4-aryl-linked bis-β-diketonato ligands. Dalton Trans. 2008, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.K.; Gloe, K.; Hayter, M.J.; Kataeva, O.; Lindoy, L.F.; Moubaraki, B.; McMurtrie, J.C.; Murray, K.S.; Schilter, D. New discrete and polymeric supramolecular architectures derived from dinuclear (bis-β-diketonato) copper(II) metallocycles. Dalton Trans. 2006, 3977–3984. [Google Scholar] [CrossRef]
- Rancan, M.; Tessarolo, J.; Zanonato, P.L.; Seraglia, R.; Quici, S.; Armelao, L. Self-assembly of a constitutional dynamic library of Cu(II) coordination polygons and reversible sorting by crystallization. Dalton Trans. 2013, 42, 7534–7538. [Google Scholar] [CrossRef]
- Rancan, M.; Dolmella, A.; Seraglia, R.; Orlandi, S.; Quici, S.; Armelao, L. A templating guest sorts out a molecular triangle from a dimer–trimer constitutional dynamic library. Chem. Commun. 2012, 48, 3115–3117. [Google Scholar] [CrossRef]
- Rancan, M.; Tessarolo, J.; Casarin, M.; Zanonato, P.L.; Quici, S.; Armelao, L. Double Level Selection in a Constitutional Dynamic Library of Coordination Driven Supramolecular Polygons. Inorg. Chem. 2014, 53, 7276–7287. [Google Scholar] [CrossRef]
- Rancan, M.; Tessarolo, J.; Quici, S.; Armelao, L. Post-assembly guest oxidation in a metallo-supramolecular host and structural rearrangement to a coordination polymer. Chem. Commun. 2014, 50, 13761–13764. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Sluch, I.M.; Miranda, A.J.; Elbjeirami, O.; Omary, M.A.; Slaughter, L.M. Interplay of Metallophilic Interactions, π–π Stacking, and Ligand Substituent Effects in the Structures and Luminescence Properties of Neutral Pt II and Pd II Aryl Isocyanide Complexes. Inorg. Chem. 2012, 51, 10728–10746. [Google Scholar] [CrossRef]
- Musumeci, C.; Osella, S.; Ferlauto, L.; Niedzialek, D.; Grisanti, L.; Bonacchi, S.; Jouaiti, A.; Milita, S.; Ciesielski, A.; Beljonne, D.; et al. Influence of the supramolecular order on the electrical properties of 1D coordination polymers based materials. Nanoscale 2016, 8, 2386–2394. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Shimada, T.; Nagumo, H.; Nabeshima, T. Highly cooperative double metalation of a bis(N2O2) ligand based on bipyridine-phenol framework driven by intramolecular π-stacking of square planar nickel(II) complex moieties. Dalton Trans. 2011, 40, 8507–8509. [Google Scholar] [CrossRef] [PubMed]
- Kammer, S.; Müller, H.; Grunwald, N.; Bellin, A.; Kelling, A.; Schilde, U.; Mickler, W.; Dosche, C.; Holdt, H.J. Supramolecular Assemblies with Honeycomb Structures by π–π Stacking of Octahedral Metal Complexes of 1,12-Diazaperylene. Eur. J. Inorg. Chem. 2006, 2006, 1547–1551. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Vujovic, S.; Zampese, J.A. Metallohexacycles containing 4′-aryl-4,2′:6′, 4′′-terpyridines: Conformational preferences and fullerene capture. CrystEngComm 2014, 16, 328–338. [Google Scholar] [CrossRef]
- Tzeng, B.C.; Lin, J.F. Crystal-engineering and luminescence studies of 1,3,5-tris (3-pyridylethynyl) benzene or 1,3,5-tris (4-pyridylethynyl) benzene with copper(I) iodides. Dalton Trans. 2019, 48, 4046–4057. [Google Scholar] [CrossRef]
- Ju, H.; Lee, E.; Kim, S.; Park, I.H.; Lee, J.H.; Lee, S.S. Cation-directed assembly of polyrotaxane and polycatenane. CrystEngComm 2016, 18, 2621–2625. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Jiang, F.L.; Wu, M.Y.; Ma, J.; Bu, Y.; Hong, M.C. Assembly of discrete one-, two-, and three-dimensional Zn(II) complexes containing semirigid V-shaped tricarboxylate ligands. Cryst. Growth Des. 2012, 12, 1452–1463. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wilseck, Z.M.; Laduca, R.L. 1D + 1D → 1D polyrotaxane, 2D + 2D → 3D interpenetrated, and 3D self-penetrated divalent metal terephthalate bis (pyridylformyl) piperazine coordination polymers. Inorg. Chem. 2011, 50, 8997–9003. [Google Scholar] [CrossRef]
- Gao, X.M.; Li, D.S.; Wang, J.J.; Fu, F.; Wu, Y.P.; Hu, H.M.; Wang, J.W. A novel 1D armed-polyrotaxane chain constructed from a V-shaped tetracarboxylate ligand. CrystEngComm 2008, 10, 479–482. [Google Scholar] [CrossRef]
- Hazari, D.; Jana, S.K.; Puschmann, H.; Zangrando, E.; Dalai, S. 1D lead(II) coordination chains with carboxylate containing ligands. A rare example of polyrotaxane 1D → 1D interpenetrated coordination polymer. Inorg. Chem. Commun. 2016, 65, 1–3. [Google Scholar] [CrossRef]




| Bond | [Zn2L2(MeOH)2], 1 | [Cu2L2(MeOH)2], 2 | [Zn2L2(EtOH)2], 3 | [Zn2L2(py)2], 5 |
|---|---|---|---|---|
| M–O1 | 2.0153(16) | 1.9338(19) | 2.0525(16) | 1.9817(13) |
| M–O2 | 1.9951(16) | 1.9404(18) | 2.0008(17) | 2.0681(12) |
| M–O3 | 2.0304(16) | 1.9430(18) | 2.0283(17) | 1.9918(13) |
| M–O4 | 2.0023(17) | 1.9290(19) | 2.092(9) | 2.0948(12) |
| M–O5 (ROH) | 2.0213(19) | 2.245(2) | 2.008(3) | --- |
| M–N1 (py) | --- | --- | --- | 2.0608(15) |
| M···M | 12.5034(9) | 12.4735(14) | 12.5255(7) | 12.8868(5) |
| C···C 1 | 11.472(5) | 11.269(6) | 11.481(4) | 11.011(3) |
| [Zn2L2(MeOH)2], 1 | [Cu2L2(MeOH)2], 2 | [Zn2L2(EtOH)2], 3 |
|---|---|---|
| d(O5H···O3) = 2.136(18) Å | d(O5H···O3) = 2.0784(2) Å | d(O5H···O1) = 1.959(12) Å |
| ∠(O5H···O3) = 144.707(4)° | ∠(O5H···O3) = 164.085(3)° | ∠(O5H···O1) = 162.41(2)° |
| d(O5H···O1) = 2.281(5) Å | d(O5H···O2) = 2.5744(3) Å | |
| ∠(O5H···O1) = 133.308(4)° | ∠(O5H···O2) = 121.359(8)° |
| Compound | 1 | 2 | 3 | 5 |
|---|---|---|---|---|
| Formula | C66H54O10Cl6Zn2 | C66H56Cl4O10Cu2 | C68H58O10Cl6Zn2 | C74H56Cl6N2O8Zn2 |
| Formula weight | 1111.79 | 1277.98 | 1139.84 | 1444.64 |
| Temperature/K | 143(1) | 139.9(2) | 150.1(2) | 143(1) |
| Crystal system | triclinic | triclinic | triclinic | triclinic |
| Space group | P-1 | P-1 | P-1 | P-1 |
| a/Å | 9.9444(5) | 10.1048(9) | 9.9945(3) | 8.8428(2) |
| b/Å | 12.9319(6) | 12.9002(11) | 12.8141(3) | 12.4056(3) |
| c/Å | 13.6843(7) | 13.1829(12) | 13.8744(4) | 15.8865(4) |
| α/° | 99.295(4) | 67.018(9) | 98.971(2) | 88.908(2) |
| β/° | 104.338(4) | 76.573(8) | 105.037(3) | 79.215(2) |
| γ/° | 111.088(5) | 67.713(8) | 110.495(2) | 74.360(2) |
| Volume/Å3 | 1528.45(14) | 1456.7(3) | 1546.47(8) | 1647.67(8) |
| Z | 1 | 1 | 1 | 1 |
| Crystal size/mm3 | 0.41 × 0.23 × 0.13 | 0.3 × 0.15 × 0.02 | 0.35 × 0.31 × 0.25 | 0.38 × 0.22 × 0.1 |
| Radiation | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) |
| 2θ range for data collection/° | 4.654–58.706 | 4.698–52.744 | 4.526–58.502 | 4.872–58.766 |
| Reflections collected | 24600 | 11109 | 19517 | 28460 |
| Independent reflections | 7322 [Rint = 0.0330, Rsigma = 0.0349] | 5954 [Rint = 0.0368, Rsigma = 0.0587] | 7268 [Rint = 0.0200, Rsigma = 0.0248] | 7994 [Rint = 0.0305, Rsigma = 0.0285] |
| Data/restraints/parameters | 7322/339/384 | 5954/3/374 | 7268/18/408 | 7994/0/415 |
| Goodness-of-fit on F2 | 1.031 | 1.027 | 1.037 | 1.029 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0465, wR2 = 0.1176 | R1 = 0.0460, wR2 = 0.1092 | R1 = 0.0483, wR2 = 0.1308 | R1 = 0.0359, wR2 = 0.0883 |
| Final R indexes [all data] | R1 = 0.0585, wR2 = 0.1263 | R1 = 0.0610, wR2 = 0.1202 | R1 = 0.0624, wR2 = 0.1424 | R1 = 0.0428, wR2 = 0.0931 |
| Largest diff. peak/hole/e Å−3 | 1.80/−0.58 | 0.45/−0.56 | 1.16/−0.88 | 0.71/−0.61 |
| CCDC | 1955909 | 1955907 | 1964015 | 1955908 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rancan, M.; Truccolo, G.; Carlotto, A.; Quici, S.; Armelao, L. A Zn(II) Metallocycle as Platform to Assemble a 1D + 1D → 1D Polyrotaxane via π···π Stacking of an Ancillary Ligand. Inorganics 2019, 7, 137. https://doi.org/10.3390/inorganics7110137
Rancan M, Truccolo G, Carlotto A, Quici S, Armelao L. A Zn(II) Metallocycle as Platform to Assemble a 1D + 1D → 1D Polyrotaxane via π···π Stacking of an Ancillary Ligand. Inorganics. 2019; 7(11):137. https://doi.org/10.3390/inorganics7110137
Chicago/Turabian StyleRancan, Marzio, Giada Truccolo, Alice Carlotto, Silvio Quici, and Lidia Armelao. 2019. "A Zn(II) Metallocycle as Platform to Assemble a 1D + 1D → 1D Polyrotaxane via π···π Stacking of an Ancillary Ligand" Inorganics 7, no. 11: 137. https://doi.org/10.3390/inorganics7110137
APA StyleRancan, M., Truccolo, G., Carlotto, A., Quici, S., & Armelao, L. (2019). A Zn(II) Metallocycle as Platform to Assemble a 1D + 1D → 1D Polyrotaxane via π···π Stacking of an Ancillary Ligand. Inorganics, 7(11), 137. https://doi.org/10.3390/inorganics7110137