High-Pressure Synthesis of Non-Stoichiometric LixWO3 (0.5 ≤ x ≤ 1.0) with LiNbO3 Structure
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of LixWO3 (0.5 ≤ x ≤ 1)
2.2. Structural Characterizations of Li0.8WO3
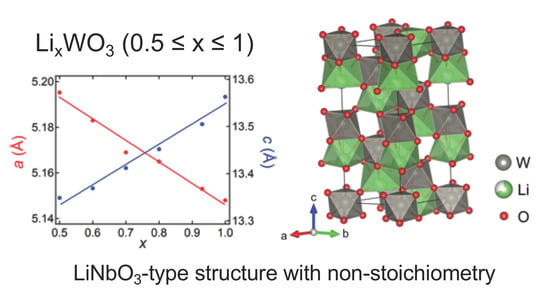
2.3. Structural Transition in LixWO3
2.4. Physical Properties
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- OK, K.M.; Chi, E.O.; Halasyamani, P.S. Bulk characterization methods for non-centrosymmetric materials: Second-harmonic generation, piezoelectricity, pyroelectricity, and ferroelectricity. Chem. Soc. Rev. 2006, 35, 710. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.R.; Sundaresan, A.; Saha, R.J. Multiferroic and magnetoelectric oxides: The emerging scenario. Phys. Chem. Lett. 2012, 3, 2237. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Fujita, K.; Yamada, I.; Matoba, T.; Kim, S.J.; Gao, P.; Pan, X.; Findlay, S.D.; Tassel, C.; Kageyama, H.; et al. Room-temperature polar ferromagnet ScFeO3 transformed from a high-pressure orthorhombic perovskite phase. J. Am. Chem. Soc. 2014, 136, 15291–15299. [Google Scholar] [CrossRef] [PubMed]
- Tassel, C.; Kuno, Y.; Goto, Y.; Yamamoto, T.; Brown, C.; Hester, J.; Fujita, K.; Higashi, M.; Abe, R.; Tanaka, K.; et al. Polar LiNbO3-type oxynitride with a helical spin order. Angew. Chem. Int. Ed. 2015, 54, 516–521. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, Y.; Wang, X.; Princep, A.J.; Khalyavin, D.; Manuel, P.; Michiue, Y.; Sato, A.; Tsuda, K.; Yu, S.; et al. A ferroelectric-like structural transition in a metal. Nat. Mater. 2013, 12, 1024–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeuchi, Y.; Takatsu, H.; Tassel, C.; Goto, Y.; Murakami, T.; Kageyama, H. High pressure synthesis of fully occupied tetragonal and cubic tungsten bronze oxides. Angew. Chem. Int. Ed. 2017, 56, 5770–5773. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, Y.; Takatsu, H.; Tassel, C.; Brown, C.M.; Murakami, T.; Matsumoto, Y.; Okamoto, Y.; Kageyama, H. Rattling behavior in a simple perovskite NaWO3. Inorg. Chem. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, P.J.; Dickens, P.G. Neutron diffraction studies of cubic tungsten bronzes. J. Solid State Chem. 1976, 17, 91. [Google Scholar] [CrossRef]
- Cava, R.J.; Santoro, A.; Murphy, D.W.; Zahurak, S.M.; Roth, R.S. The structures of the lithium inserted metal oxides Li0.2ReO3 and Li0.36WO3. J. Solid State Chem. 1983, 50, 121–128. [Google Scholar] [CrossRef]
- Rahman, M.S.; Murshed, M.M.; Gesing, T.M. Synthesis, characterization and time dependent phase transformation of Li0.4WO3 bronze. Crystalline Mater. 2014, 229, 797–805. [Google Scholar] [CrossRef]
- Zhong, Q.; Dahn, J.R.; Colbow, K. Lithium intercalation into WO3 and the phase diagram of LixWO3. Phys. Rev. B 1992, 46, 2554. [Google Scholar] [CrossRef]
- Nishihaya, S.; Uchida, M.; Kozuka, Y.; Iwasa, Y.; Kawasaki, M. Evolution of insulator–metal phase transitions in epitaxial tungsten oxide films during electrolyte-gating. ACS Appl. Mater. Interfaces 2016, 8, 22330–22336. [Google Scholar] [CrossRef] [PubMed]
- Morkoç, H.; Özgür, Ü. Zinc Oxide: Fundamentals, Materials and Device Technology; Wiley: New York, NY, USA, 2009. [Google Scholar]
- Abrahams, S.C.; Bernstein, J.L. Ferroelectric lithium tantalate—1. Single crystal X-ray diffraction study at 24 °C. J. Phys. Chem. Solids 1967, 28, 1685–1692. [Google Scholar] [CrossRef]
- Boysen, H.; Altorfer, F. A neutron powder investigation of the high-temperature structure and phase transition in LiNbO3. Acta Crystallogr. B 1994, 50, 405–414. [Google Scholar] [CrossRef]
- Xiang, H.J. Origin of polar distortion in LiNbO3-type “ferroelectric” metals: Role of A-site instability and short-range interactions. Phys. Rev. B 2014, 90, 094108. [Google Scholar] [CrossRef]
- Giovannetti, G.; Capone, M. Dual nature of the ferroelectric and metallic state in LiOsO3. Phys. Rev. B 2014, 90, 195113. [Google Scholar] [CrossRef]
- Jin, F.; Zhang, A.; Ji, J.; Liu, K.; Wang, L.; Shi, Y.; Tian, Y.; Ma, X.; Zhang, Q. Raman phonons in the ferroelectric-like metal LiOsO3. Phys. Rev. B 2016, 93, 064303. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Marsh, P. Defect structure dependence on composition in lithium niobate. Acta Crystallogr. B 1986, 42, 61–68. [Google Scholar] [CrossRef] [Green Version]




| Technique | Atom | Wyckoff Position | g | x | y | z | Uiso (Å2) |
|---|---|---|---|---|---|---|---|
| XRD | Li | 12c | 1 | 0 | 0 | 0.2502(6) | 0 |
| W | 6b | 1 | 0 | 0 | 0 | 0 | |
| O | 18e | 1 | 0.076(3) | 0.371(6) | 0.0829(2) | 0 | |
| ND | Li | 12c | 0.77(5) | 0 | 0 | 0.2748(16) | 0.014(6) |
| W | 6b | 1 | 0 | 0 | 0 | 0.0034(8) | |
| O | 18e | 1 | 0.0659(12) | 0.332(2) | 0.0821(14) | 0.0063(5) |
| x | 0.5 | 0.6 | 0.7 | 0.8 | 0.85 | 1.0 |
|---|---|---|---|---|---|---|
| Pressure (GPa) | 2 | 5 | 5 | 6 | 6 | 8 |
| Temperature (°C) | 850 | 850 | 1000 | 1200 | 1200 | 1200 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishida, K.; Ikeuchi, Y.; Tassel, C.; Takatsu, H.; Brown, C.M.; Kageyama, H. High-Pressure Synthesis of Non-Stoichiometric LixWO3 (0.5 ≤ x ≤ 1.0) with LiNbO3 Structure. Inorganics 2019, 7, 63. https://doi.org/10.3390/inorganics7050063
Ishida K, Ikeuchi Y, Tassel C, Takatsu H, Brown CM, Kageyama H. High-Pressure Synthesis of Non-Stoichiometric LixWO3 (0.5 ≤ x ≤ 1.0) with LiNbO3 Structure. Inorganics. 2019; 7(5):63. https://doi.org/10.3390/inorganics7050063
Chicago/Turabian StyleIshida, Kohdai, Yuya Ikeuchi, Cédric Tassel, Hiroshi Takatsu, Craig M. Brown, and Hiroshi Kageyama. 2019. "High-Pressure Synthesis of Non-Stoichiometric LixWO3 (0.5 ≤ x ≤ 1.0) with LiNbO3 Structure" Inorganics 7, no. 5: 63. https://doi.org/10.3390/inorganics7050063
APA StyleIshida, K., Ikeuchi, Y., Tassel, C., Takatsu, H., Brown, C. M., & Kageyama, H. (2019). High-Pressure Synthesis of Non-Stoichiometric LixWO3 (0.5 ≤ x ≤ 1.0) with LiNbO3 Structure. Inorganics, 7(5), 63. https://doi.org/10.3390/inorganics7050063