The Effect of Storage-Induced Changes in Ammonia Borane on Hydrogen Release during Its Low-Temperature Thermolysis
Abstract
:1. Introduction
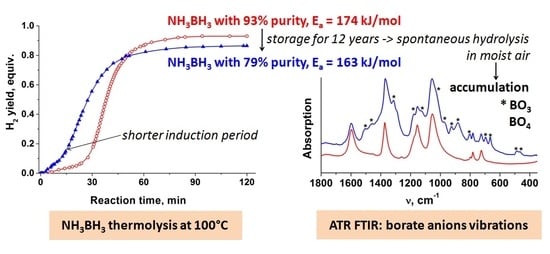
2. Results
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, P. Solid-state thermolysis of ammonia borane and related materials for high-capacity hydrogen storage. Dalt. Trans. 2012, 41, 4296. [Google Scholar] [CrossRef]
- Demirci, U.B. Ammonia borane, a material with exceptional properties for chemical hydrogen storage. Int. J. Hydrog. Energy 2017, 42, 9978–10013. [Google Scholar] [CrossRef]
- Baitalow, F.; Baumann, J.; Wolf, G.; Jaenicke-Rößler, K.; Leitner, G. Thermal decomposition of B–N–H compounds investigated by using combined thermoanalytical methods. Thermochim. Acta 2002, 391, 159–168. [Google Scholar] [CrossRef]
- Frueh, S.; Kellett, R.; Mallery, C.; Molter, T.; Willis, W.S.; King’ondu, C.; Suib, S.L. Pyrolytic decomposition of ammonia borane to boron nitride. Inorg. Chem. 2011, 50, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Al-Kukhun, A.; Hwang, H.T.; Varma, A. Mechanistic studies of ammonia borane dehydrogenation. Int. J. Hydrog. Energy 2013, 38, 169–179. [Google Scholar] [CrossRef]
- Petit, J.-F.; Demirci, U.B. Mechanistic insights into dehydrogenation of partially deuterated ammonia borane NH3BD3 being heating to 200 °C. Inorg. Chem. 2019, 58, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Nguyen, V.S.; Matus, M.H.; Gopakumar, G.; Dixon, D.A. Molecular mechanism for H2 release from BH3NH3, including the catalytic role of the Lewis acid BH3. J. Phys. Chem. A 2007, 111, 679–690. [Google Scholar] [CrossRef]
- He, T.; Xiong, Z.; Wu, G.; Chu, H.; Wu, C.; Zhang, T.; Chen, P. Nanosized Co- and Ni-catalyzed ammonia borane for hydrogen storage. Chem. Mater. 2009, 21, 2315–2318. [Google Scholar] [CrossRef]
- Ergüven, H.; Kantürk Figen, A.; Pişkin, S. Ammonia borane–boron composites for hydrogen release: Thermolysis kinetics. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 613–617. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, D.; Chen, B.; Liu, Z.; Xia, Q.; Zhu, Y.; Xia, Y. Improved hydrogen release from ammonia borane confined in microporous carbon with narrow pore size distribution. J. Mater. Chem. A 2017, 5, 15395–15400. [Google Scholar] [CrossRef]
- Gangal, A.C.; Sharma, P. Kinetic analysis and modeling of thermal decomposition of ammonia borane. Int. J. Chem. Kinet. 2013, 45, 452–461. [Google Scholar] [CrossRef]
- Petit, J.-F.; Miele, P.; Demirci, U.B. Ammonia borane H3N-BH3 for solid-state chemical hydrogen storage: Different samples with different thermal behaviors. Int. J. Hydrog. Energy 2016, 41, 15462–15470. [Google Scholar] [CrossRef]
- Keskin, E.; Coşkuner Filiz, B.; Kılıç Depren, S.; Kantürk Figen, A. Recommendations for ammonia borane composite pellets as a hydrogen storage medium. Int. J. Hydrog. Energy 2018, 43, 20354–20371. [Google Scholar] [CrossRef]
- Simagina, V.I.; Vernikovskaya, N.V.; Komova, O.V.; Kayl, N.L.; Netskina, O.V.; Odegova, G.V. Experimental and modeling study of ammonia borane-based hydrogen storage systems. Chem. Eng. J. 2017, 329, 156–164. [Google Scholar] [CrossRef]
- Komova, O.V.; Simagina, V.I.; Kayl, N.L.; Odegova, G.V.; Netskina, O.V.; Chesalov, Y.A.; Ozerova, A.M. Improved low-temperature hydrogen generation from NH3BH3 and TiO2 composites pretreated with water. Int. J. Hydrog. Energy 2013, 38, 6442–6449. [Google Scholar] [CrossRef]
- Shaw, W.J.; Bowden, M.; Karkamkar, A.; Howard, C.J.; Heldebrant, D.J.; Hess, N.J.; Linehan, J.C.; Autrey, T. Characterization of a new phase of ammonia borane. Energy Environ. Sci. 2010, 3, 796. [Google Scholar] [CrossRef]
- Staubitz, A.; Robertson, A.P.M.; Manners, I. Ammonia-borane and related compounds as dihydrogen sources. Chem. Rev. 2010, 110, 4079–4124. [Google Scholar] [CrossRef]
- Stowe, A.C.; Shaw, W.J.; Linehan, J.C.; Schmid, B.; Autrey, T. In situ solid state 11B MAS-NMR studies of the thermal decomposition of ammonia borane: Mechanistic studies of the hydrogen release pathways from a solid state hydrogen storage material. Phys. Chem. Chem. Phys. 2007, 9, 1831. [Google Scholar] [CrossRef]
- Yamada, K. A solid-state NMR study of boric acid doped in poly(vinyl alcohol). In NMR Spectroscopy of Polymers: Innovative Strategies for Complex Macromolecules; Cheng, H.N., Asakura, T., English, A.D., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011; pp. 133–146. [Google Scholar] [CrossRef]
- Liu, C.-H.; Wu, Y.-C.; Chou, C.-C.; Chen, B.-H.; Hsueh, C.-L.; Ku, J.-R.; Tsau, F. Hydrogen generated from hydrolysis of ammonia borane using cobalt and ruthenium based catalysts. Int. J. Hydrog. Energy 2012, 37, 2950–2959. [Google Scholar] [CrossRef]
- Mohajeri, N.; T-Raissi, A.; Adebiyi, O. Hydrolytic cleavage of ammonia-borane complex for hydrogen production. J. Power Sources 2007, 167, 482–485. [Google Scholar] [CrossRef]
- Chou, C.-C.; Lee, D.-J.; Chen, B.-H. Hydrogen production from hydrolysis of ammonia borane with limited water supply. Int. J. Hydrog. Energy 2012, 37, 15681–15690. [Google Scholar] [CrossRef]
- Moussa, G.; Moury, R.; Demirci, U.B.; Miele, P. Borates in hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2013, 38, 7888–7895. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Liang, J.; Chen, J. Hydrogen releasing of lithium amidoborane-LiNH2BH3. Mater. Trans. 2011, 52, 651–653. [Google Scholar] [CrossRef]
- Xie, S.; Song, Y.; Liu, Z. In situ high-pressure study of ammonia borane by Raman and IR spectroscopy. Can. J. Chem. 2009, 87, 1235–1247. [Google Scholar] [CrossRef]
- Jiang, W.; Li-Xia, Z.; Shu-Ping, X.; Bo, W.; Shi-Yang, G. FTIR and Raman spectroscopic study of hydrated ammonium borates and their saturated aqueous solutions. Chem. J. Chin. Univ. 2004, 25, 1213–1217. [Google Scholar]
- Bertoluzza, A.; Monti, P.; Battaglia, M.A.; Bonora, S. Infrared and raman spectra of orthorhombic, monoclinic and cubic metaboric acid and their relation to the “strength” of the hydrogen bond present. J. Mol. Struct. 1980, 64, 123–136. [Google Scholar] [CrossRef]
- Gutowska, A.; Li, L.; Shin, Y.; Wang, C.M.; Li, X.S.; Linehan, J.C.; Smith, R.S.; Kay, B.D.; Schmid, B.; Shaw, W.; et al. Nanoscaffold mediates hydrogen release and the reactivity of ammonia borane. Angew. Chemie Int. Ed. 2005, 44, 3578–3582. [Google Scholar] [CrossRef]
- Sepehri, S.; Garcia, B.B.; Cao, G. Tuning dehydrogenation temperature of carbon-ammonia borane nanocomposites. J. Mater. Chem. 2008, 18, 4034. [Google Scholar] [CrossRef]
- Kang, X.; Fang, Z.; Kong, L.; Cheng, H.; Yao, X.; Lu, G.; Wang, P. Ammonia borane destabilized by lithium hydride: An advanced on-board hydrogen storage material. Adv. Mater. 2008, 20, 2756–2759. [Google Scholar] [CrossRef]
- Si, X.; Sun, L.; Xu, F.; Jiao, C.; Li, F.; Liu, S.; Zhang, J.; Song, L.; Jiang, C.; Wang, S.; et al. Improved hydrogen desorption properties of ammonia borane by Ni-modified metal-organic frameworks. Int. J. Hydrog. Energy 2011, 36, 6698–6704. [Google Scholar] [CrossRef]
- Komova, O.V.; Kayl, N.L.; Odegova, G.V.; Netskina, O.V.; Simagina, V.I. Destabilization of NH3BH3 by water during hydrothermolysis as a key factor in the high hydrogen evolution rates. Int. J. Hydrog. Energy 2016, 41, 17484–17495. [Google Scholar] [CrossRef]
- Hwang, H.T.; Varma, A. Effect of boric acid on thermal dehydrogenation of ammonia borane: Mechanistic studies. Int. J. Hydrog. Energy 2013, 38, 1925–1931. [Google Scholar] [CrossRef]
- Roy, A.; Choudhury, A.; Rao, C.N.R. Supramolecular hydrogen-bonded structure of a 1:2 adduct of melamine with boric acid. J. Mol. Struct. 2002, 613, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Butlak, A.V.; Kondrat’ev, Y.V.; Timoshkin, A.Y. Determination of the enthalpy of ammonia borane sublimation. Russ. J. Gen. Chem. 2014, 84, 2455–2456. [Google Scholar] [CrossRef]
- Mohajeri, N.; T-Raissi, A.; Ramasamy, K.K. Thermal conductivity of ammonia borane complex and its composites with aluminum powder. Thermochim. Acta 2007, 452, 28–30. [Google Scholar] [CrossRef]
- Petit, J.-F.; Demirci, U.B. Discrepancy in the thermal decomposition/dehydrogenation of ammonia borane screened by thermogravimetric analysis. Int. J. Hydrog. Energy 2019, 44, 14201–14206. [Google Scholar] [CrossRef]
- Gorlova, A.M.; Kayl, N.L.; Komova, O.V.; Netskina, O.V.; Ozerova, A.M.; Odegova, G.V.; Bulavchenko, O.A.; Ishchenko, A.V.; Simagina, V.I. Fast hydrogen generation from solid NH3BH3 under moderate heating and supplying a limited quantity of CoCl2 or NiCl2 solution. Renew. Energy 2018, 121, 722–729. [Google Scholar] [CrossRef]
- Ozerova, A.M.; Bulavchenko, O.A.; Komova, O.V.; Netskina, O.V.; Zaikovskii, V.I.; Odegova, G.V.; Simagina, V.I. Cobalt boride catalysts for hydrogen storage systems based on NH3BH3 and NaBH4. Kinet. Catal. 2012, 53, 511–520. [Google Scholar] [CrossRef]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Calvé, S.; Alonso, B.; Durand, J.-O.; Bujoli, B.; Gan, Z.; Hoatson, G. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]






| Borates Absorption Bands | NH4B5O8·4H2O (B(3)–O, B(4)–O) [26] | (NH4)2B8O13·6H2O (B(3)–O, B(4)–O) [26] | HBO2 Monoclinic (B(3)–O, B(4)–O) [27] | HBO2 Cubic (B(4)–O) [27] |
|---|---|---|---|---|
| 1460 | 1436 s, b | 1468 s, b 1398 s, b | 1485 sh 1400 vs, b | 1463 vs, b |
| 1312 | 1351 s, b | 1346 s, b | 1335 vs 1318 sh | |
| 1290 | 1238 m | 1290 sh 1205 s | ||
| 1179 1125 | 1182 m 1113 w | 1124 vs | 1183 vs, b | |
| 975 925 | 921 s | 969 m 923 s | 980 w 960 m 918 w | 993 vs, b 945 vs, b |
| 881 | 884 m | 877 s | 895 m, b | |
| 805 | 781 s | 806 m | 803 m | 825 vs, b |
| 696 | 692 s | 696 m | 690 m | 680 sh |
| 665 | 642 m | 666 s | 653 m 620 wv | 643 s, sh |
| 488 | 459 w | 486 s, sh | 481 m 443 m 423 m | 742 s 433 m, b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komova, O.V.; Netskina, O.V.; Ozerova, A.M.; Odegova, G.V.; Arzumanov, S.S.; Simagina, V.I. The Effect of Storage-Induced Changes in Ammonia Borane on Hydrogen Release during Its Low-Temperature Thermolysis. Inorganics 2019, 7, 96. https://doi.org/10.3390/inorganics7080096
Komova OV, Netskina OV, Ozerova AM, Odegova GV, Arzumanov SS, Simagina VI. The Effect of Storage-Induced Changes in Ammonia Borane on Hydrogen Release during Its Low-Temperature Thermolysis. Inorganics. 2019; 7(8):96. https://doi.org/10.3390/inorganics7080096
Chicago/Turabian StyleKomova, Oxana V., Olga V. Netskina, Anna M. Ozerova, Galina V. Odegova, Sergei S. Arzumanov, and Valentina I. Simagina. 2019. "The Effect of Storage-Induced Changes in Ammonia Borane on Hydrogen Release during Its Low-Temperature Thermolysis" Inorganics 7, no. 8: 96. https://doi.org/10.3390/inorganics7080096
APA StyleKomova, O. V., Netskina, O. V., Ozerova, A. M., Odegova, G. V., Arzumanov, S. S., & Simagina, V. I. (2019). The Effect of Storage-Induced Changes in Ammonia Borane on Hydrogen Release during Its Low-Temperature Thermolysis. Inorganics, 7(8), 96. https://doi.org/10.3390/inorganics7080096