Polymer Coated Semiconducting Nanoparticles for Hybrid Materials
Abstract
:1. Introduction
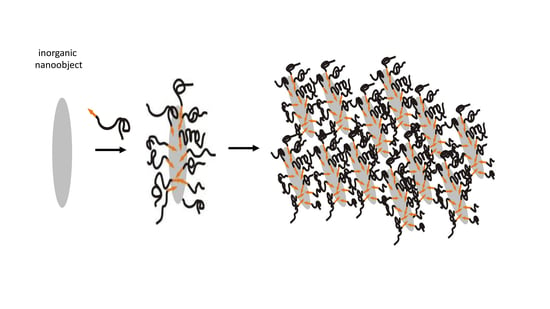
2. Concepts for the Functionalization of Inorganic Nanoparticles to Improve Their Solubility and Processability
3. Controlling Orientation, Dispersion, and Percolation of Inorganic Nanoparticles in a Polymer Matrix
4. QD-LEDs
5. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Vaia, R.A.; Wagner, H.D. Framework for nanocomposites. Mater. Today 2004, 7, 32–37. [Google Scholar] [CrossRef]
- Bolhuis, P.G.; Kofke, D.A. Monte carlo study of freezing of polydisperse hard spheres. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 1996, 54, 634–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, P.; Warren, P.B. Reentrant melting in polydispersed hard spheres. Phys. Rev. Lett. 1999, 82, 1979–1982. [Google Scholar] [CrossRef]
- Fleischhaker, F.; Zentel, R. Photonic crystals from core-shell colloids with incorporated highly fluorescent quantum dots. Chem. Mater. 2005, 17, 1346–1351. [Google Scholar] [CrossRef]
- Stegemeyer, H.; Behret, H. (Eds.) Liquid Crystals; Steinkopff: Heidelberg, Germany, 1994. [Google Scholar] [CrossRef]
- Demus, D.; Goodby, J.; Gray, G.W.; Spiess, H.-W.; Vill, V. Handbook of Liquid Crystals; Wiley: Hoboken, NJ, USA, 1998. [Google Scholar] [CrossRef]
- Fischer, S.; Salcher, A.; Kornowski, A.; Weller, H.; Förster, S. Completely miscible nanocomposites. Angew. Chem. Int. Ed. 2011, 50, 7811–7814. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J. Intermolecular and Surface Forces, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Ehlert, S.; Taheri, S.M.; Pirner, D.; Drechsler, M.; Schmidt, H.-W.; Förster, S. Polymer ligand exchange to control stabilization and compatibilization of nanocrystals. ACS Nano 2014, 8, 6114–6122. [Google Scholar] [CrossRef]
- Butt, H.-J.; Kappl, M. Surface and Interfacial Forces 2e; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018. [Google Scholar] [CrossRef]
- Bredol, M.; Matras, K.; Szatkowski, A.; Sanetra, J.; Prodi-Schwab, A. P3HT/ZnS: A New hybrid bulk heterojunction photovoltaic system with very high open circuit voltage. Sol. Energy Mater. Sol. Cells 2009, 93, 662–666. [Google Scholar] [CrossRef]
- Guchhait, A.; Rath, A.K.; Pal, A.J. To make polymer: Quantum dot hybrid solar cells NIR-active by increasing diameter of PbSnanoparticles. Sol. Energy Mater. Sol. Cells 2011, 95, 651–656. [Google Scholar] [CrossRef]
- Ren, S.; Chang, L.Y.; Lim, S.K.; Zhao, J.; Smith, M.; Zhao, N.; Bulović, V.; Bawendi, M.; Gradečak, S. Inorganic-organic hybrid solar cell: Bridging quantum dots to conjugated polymer nanowires. Nano Lett. 2011, 11, 3998–4002. [Google Scholar] [CrossRef]
- Ballauff, M. Stiff-Chain Polymers-Structure, Phase Behaviour, and Properties. Angew. Chem. Int. Ed. 1989, 59, 253–267. [Google Scholar] [CrossRef]
- Li, L.S.; Walda, J.; Manna, L.; Alivisatos, A.P. Semiconductor nanorod liquid crystals. Nano Lett. 2002, 2. [Google Scholar] [CrossRef] [Green Version]
- Jana, N.R. Nanorod shape separation using surfactant assisted self-assembly. Chem. Commun. 2003, 9, 1950–1951. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, B.J.; Davidson, P.; Ferré, J.; Jamet, J.P.; Petermann, D.; Panine, P.; Dozov, I.; Stoenescu, D.; Jolivet, J.P. The complex phase behaviour of suspensions of goethite (α-FeOOH) nanorods in a magnetic field. Faraday Discuss. 2005, 128, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Alonso, M.; McCarthy, T.J. Chemical surface modification of Poly(p-Xylylene) thin films. Langmuir 2004, 20, 9184–9189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Van Duijneveldt, J.S. Isotropic-nematic phase transition of nonaqueous suspensions of natural clay rods. J. Chem. Phys. 2006, 124. [Google Scholar] [CrossRef]
- Meuer, S.; Oberle, P.; Theato, P.; Tremel, W.; Zentel, R. Liquid crystalline phases from polymer-functionalized TiO2 nanorods. Adv. Mater. 2007, 19, 2073–2078. [Google Scholar] [CrossRef]
- Zorn, M.; Meuer, S.; Tahir, M.N.; Khalavka, Y.; Sönnichsen, C.; Tremel, W.; Zentel, R. Liquid crystalline phases from polymer functionalised semiconducting nanorods. J. Mater. Chem. 2008, 18, 3050–3058. [Google Scholar] [CrossRef]
- Van Bruggen, M.P.B.; Van Der Kooij, F.M.; Lekkerkerker, H.N.W. Liquid crystal phase transitions in dispersions of rod-like colloidal particles. J. Phys. Condens. Matter 1996, 8, 9451–9456. [Google Scholar] [CrossRef] [Green Version]
- Van Der Beek, D.; Reich, H.; Van Der Schoot, P.; Dijkstra, M.; Schilling, T.; Vink, R.; Schmidt, M.; Van Roij, R.; Lekkerkerker, H. Isotropic-nematic interface and wetting in suspensions of colloidal platelets. Phys. Rev. Lett. 2006, 97, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Mathias, F.; Fokina, A.; Landfester, K.; Tremel, W.; Schmid, F.; Char, K.; Zentel, R. Morphology control in biphasic hybrid systems of semiconducting materials. Macromol. Rapid Commun. 2015, 36, 959–983. [Google Scholar] [CrossRef]
- Fokina, A.; Lee, Y.; Chang, J.H.; Park, M.; Sung, Y.; Bae, W.K.; Char, K.; Lee, C.; Zentel, R. The role of emission layer morphology on the enhanced performance of light-emitting diodes based on quantum dot-semiconducting polymer hybrids. Adv. Mater. Interfaces 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Skaff, H.; Ilker, M.F.; Coughlin, E.B.; Emrick, T. Preparation of cadmium selenide-polyolefin composites from functional phosphine oxides and ruthenium-based metathesis. J. Am. Chem. Soc. 2002, 124, 5729–5733. [Google Scholar] [CrossRef] [PubMed]
- Javier, A.; Yun, C.S.; Sorena, J.; Strouse, G.F. Energy transport in CdSe nanocrystals assembled with molecular wires. J. Phys. Chem. B 2003, 107, 435–442. [Google Scholar] [CrossRef]
- Sill, K.; Emrick, T. Nitroxide-mediated radical polymerization from CdSe nanoparticles. Chem. Mater. 2004, 16, 1240–1243. [Google Scholar] [CrossRef]
- Skaff, H.; Emrick, T. Reversible addition fragmentation chain transfer (RAFT) polymerization from unprotected cadmium selenide nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 5383–5386. [Google Scholar] [CrossRef] [PubMed]
- Esteves, A.C.C.; Bombalski, L.; Trindade, T.; Matyjaszewski, K.; Barros-Timmons, A. Polymer grafting from CdS quantum dots via AGET ATRP in miniemulsion. Small 2007, 3, 1230–1236. [Google Scholar] [CrossRef]
- Zhang, Q.; Russell, T.P.; Emrick, T. Synthesis and characterization of CdSe nanorods functionalized with regioregular Poly(3-Hexylthiophene). Chem. Mater. 2007, 19, 3712–3716. [Google Scholar] [CrossRef]
- Sih, B.C.; Wolf, M.O. CdSe nanorods functionalized with thiol-anchored oligothiophenes. J. Phys. Chem. C 2007, 111, 17184–17192. [Google Scholar] [CrossRef]
- Sudeep, P.K.; Early, K.T.; McCarthy, K.D.; Odoi, M.Y.; Barnes, M.D.; Emrick, T. Monodisperse oligo (Phenylene vinylene) ligands on CdSe quantum dots: Synthesis and polarization anisotropy measurements. J. Am. Chem. Soc. 2008, 130, 2384–2385. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Hsu, J.-H.; Liao, Y.-C.; Yen, W.-C.; Li, S.-S.; Lin, S.-T.; Chen, C.-W.; Su, W.-F. Employing an amphiphilic interfacial modifier to enhance the performance of a Poly(3-Hexyl Thiophene)/TiO2 hybrid solar cell. J. Mater. Chem. 2011, 21, 4450. [Google Scholar] [CrossRef]
- Stalder, R.; Xie, D.; Zhou, R.; Xue, J.; Reynolds, J.R.; Schanze, K.S. Variable-gap conjugated oligomers grafted to CdSe nanocrystals. Chem. Mater. 2012, 24, 3143–3152. [Google Scholar] [CrossRef]
- Eberhardt, M.; Théato, P. Raft polymerization of pentafluorophenyl methacrylate: Preparation of reactive linear Diblock copolymers. Macromol. Rapid Commun. 2005, 26, 1488–1493. [Google Scholar] [CrossRef]
- Eberhardt, M.; Mruk, R.; Zentel, R.; Théato, P. Synthesis of pentafluorophenyl(Meth)Acrylate polymers: New precursor polymers for the synthesis of multifunctional materials. Eur. Polym. J. 2005, 41, 1569–1575. [Google Scholar] [CrossRef]
- Lim, J.; Zur Borg, L.; Dolezel, S.; Schmid, F.; Char, K.; Zentel, R. Strategy for good dispersion of well-defined tetrapods in semiconducting polymer matrices. Macromol. Rapid Commun. 2014, 35, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Meuer, S.; Fischer, K.; Mey, I.; Janshoff, A.; Schmidt, M.; Zentel, R. Liquid Crystals from Polymer-Functionalized TiO 2 nanorod mesogens. Macromolecules 2008, 41, 7946–7952. [Google Scholar] [CrossRef]
- Akcora, P.; Liu, H.; Kumar, S.K.; Moll, J.; Li, Y.; Benicewicz, B.C.; Schadler, L.S.; Acehan, D.; Panagiotopoulos, A.Z.; Pryamitsyn, V.; et al. Anisotropic self-assembly of spherical polymer-grafted nanoparticles. Nat. Mater. 2009, 8, 354–359. [Google Scholar] [CrossRef]
- Nikolic, M.S.; Olsson, C.; Saldier, A.; Kornowski, A.; Rank, A.; Schubert, R.; Frömsdorf, A.; Weller, H.; Förster, S. Micelle and vesicle formation of amphiphilic nanopartieles. Angew. Chem. Int. Ed. 2009, 48, 2752–2754. [Google Scholar] [CrossRef]
- Manna, L.; Scher, E.C.; Paul Alivisatos, A. Shape control of colloidal semiconductor nanocrystals. J. Clust. Sci. 2002, 13, 521–532. [Google Scholar] [CrossRef]
- Peng, X. Mechanisms for the shape-control and shape-evolution of colloidal semiconductor nanocrystals. Adv. Mater. 2003, 15, 459–463. [Google Scholar] [CrossRef]
- Pellegrino, T.; Manna, L.; Kudera, S.; Liedl, T.; Koktysh, D.; Rogach, A.L.; Keller, S.; Rädler, J.; Natile, G.; Parak, W.J. Hydrophobic nanocrystals coated with an amphiphilic polymer shell: A general route to water soluble nanocrystals. Nano Lett. 2004, 4, 703–707. [Google Scholar] [CrossRef]
- Yin, Y.; Alivisatos, A.P. Colloidal nanocrystal synthesis and the organic–Inorganic interface. Nature 2005, 437, 664–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Wang, J.; Lin, Z. Semiconducting nanocrystals, conjugated polymers, and conjugated polymer/nanocrystal nanohybrids and their usage in solar cells. Front. Chem. China 2010, 5, 33–44. [Google Scholar] [CrossRef]
- Li, L.S.; Alivisatos, A.P. Semiconductor nanorod liquid crystals and their assembly on a substrate. Adv. Mater. 2003, 15, 408–411. [Google Scholar] [CrossRef] [Green Version]
- Ghezelbash, A.; Koo, B.; Korgel, B.A. Self-assembled stripe patterns of CdS nanorods. Nano Lett. 2006, 6, 1832–1836. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.M.; Mastroianni, A.; Stancil, K.A.; Liu, H.; Alivisatos, A.P. Electric-field-assisted assembly of perpendicularly oriented nanorod superlattices. Nano Lett. 2006, 6, 1479–1482. [Google Scholar] [CrossRef] [Green Version]
- Querner, C.; Fischbein, M.D.; Heiney, P.A.; Drndić, M. Millimeter-scale assembly of CdSe nanorods into smectic superstructures by solvent drying kinetics. Adv. Mater. 2008, 20, 2308–2314. [Google Scholar] [CrossRef]
- Kang, C.C.; Lai, C.W.; Peng, H.C.; Shyue, J.J.; Chou, P.T. 2D self-bundled CdS nanorods with micrometer dimension in the absence of an external directing process. ACS Nano 2008, 2, 750–756. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Regulacio, M.D.; Han, M.Y. Self-assembly of colloidal one-dimensional nanocrystals. Chem. Soc. Rev. 2014, 43, 2301–2323. [Google Scholar] [CrossRef]
- Brus, L.E. Electron-electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef] [Green Version]
- Alivisatos, A.P. Semiconductor clusters, nanocrystals, and quantum dots. Science (80-.) 1996, 271, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Dabbousi, B.O.; Rodriguez-Viejo, J.; Mikulec, F.V.; Heine, J.R.; Mattoussi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (CdSe)ZnS core-shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. J. Phys. Chem. B 1997, 101, 9463–9475. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Norberg, N.S.; Nguyen, Q.P.; Parker, J.M.; Gamelin, D.R. Magnetic quantum dots: Synthesis, spectroscopy, and magnetism of Co2+- and Ni2+-doped ZnO nanocrystals. J. Am. Chem. Soc. 2003, 125, 13205–13218. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.V. Quantum dot solar cells. Semiconductor nanocrystals as light harvesters. J. Phys. Chem. C 2008, 112, 18737–18753. [Google Scholar] [CrossRef]
- Yang, P.; Lieber, C.M. Nanorod-superconductor composites: A pathway to materials with high critical current densities. Science (80-.) 1996, 273, 1836–1840. [Google Scholar] [CrossRef]
- Jun, Y.W.; Lee, S.M.; Kang, N.J.; Cheon, J. Controlled synthesis of multi-armed CdS nanorod architectures using monosurfactant system. J. Am. Chem. Soc. 2001, 123, 5150–5151. [Google Scholar] [CrossRef]
- Greene, L.E.; Law, M.; Tan, D.H.; Montano, M.; Goldberger, J.; Somorjai, G.; Yang, P. General route to vertical Zno nanowire arrays using textured ZnO seeds. Nano Lett. 2005, 5, 1231–1236. [Google Scholar] [CrossRef]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold nanorods: Synthesis, characterization and applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Manna, L.; Scher, E.C.; Alivisatos, A.P. Synthesis of soluble and processable rod-, arrow-, teardrop-, and tetrapod-shaped CdSe nanocrystals. J. Am. Chem. Soc. 2000, 122, 12700–12706. [Google Scholar] [CrossRef]
- Manna, L.; Milliron, D.J.; Meisel, A.; Scher, E.C.; Alivisatos, A.P. Controlled growth of tetrapod-branched inorganic nanocrystals. Nat. Mater. 2003, 2, 382–385. [Google Scholar] [CrossRef]
- Fiore, A.; Mastria, R.; Lupo, M.G.; Lanzani, G.; Giannini, C.; Carlino, E.; Morello, G.; De Giorgi, M.; Li, Y.; Cingolani, R.; et al. Tetrapod-shaped colloidal nanocrystals of II−VI semiconductors prepared by seeded growth. J. Am. Chem. Soc. 2009, 131, 2274–2282. [Google Scholar] [CrossRef]
- Lim, J.; Bae, W.K.; Park, K.U.; Zur Borg, L.; Zentel, R.; Lee, S.; Char, K. Controlled synthesis of CdSe tetrapods with high morphological uniformity by the persistent kinetic growth and the halide-mediated phase transformation. Chem. Mater. 2013, 25, 1443–1449. [Google Scholar] [CrossRef]
- Avis, C.; Jang, J. High-performance solution processed oxide TFT with aluminum oxide gate dielectric fabricated by a sol-gel method. J. Mater. Chem. 2011, 21, 10649–10652. [Google Scholar] [CrossRef]
- Yang, T.; Gordon, Z.D.; Chan, C.K. Synthesis of hyperbranched perovskite nanostructures. Cryst. Growth Des. 2013, 13, 3901–3907. [Google Scholar] [CrossRef]
- Li, H.; Kanaras, A.G.; Manna, L. Colloidal branched semiconductor nanocrystals: State of the art and perspectives. Acc. Chem. Res. 2013, 46, 1387–1396. [Google Scholar] [CrossRef]
- Lauhon, L.J.; Gudiksen, M.S.; Wang, D.; Lieber, C.M. Epitaxial core–shell and core–multishell nanowire heterostructures. Nature 2002, 420, 57–61. [Google Scholar] [CrossRef]
- Milliron, D.; Hughes, S.M.; Cui, Y.; Manna, L.; Li, J.; Wang, L.W.; Alivisatos, A.P. Colloidal nanocrystal heterostructures with linear and branched topology. Nature 2004, 430, 190–195. [Google Scholar] [CrossRef]
- Cho, K.S.; Talapin, D.V.; Gaschler, W.; Murray, C.B. Designing PbSe nanowires and nanorings through oriented attachment of nanoparticles. J. Am. Chem. Soc. 2005, 127, 7140–7147. [Google Scholar] [CrossRef]
- Dayal, S.; Kopidakis, N.; Olson, D.C.; Ginley, D.S.; Rumbles, G. Photovoltaic devices with a low band gap polymer and CdSe nanostructures exceeding 3% efficiency. Nano Lett. 2010, 10, 239–242. [Google Scholar] [CrossRef]
- Lee, J.S.; Kovalenko, M.V.; Huang, J.; Chung, D.S.; Talapin, D.V. Band-like transport, high electron mobility and high photoconductivity in all-inorganic nanocrystal arrays. Nat. Nanotechnol. 2011, 6, 348–352. [Google Scholar] [CrossRef]
- Jeltsch, K.F.; Schädel, M.; Bonekamp, J.B.; Niyamakom, P.; Rauscher, F.; Lademann, H.W.A.; Dumsch, I.; Allard, S.; Scherf, U.; Meerholz, K. Efficiency enhanced hybrid solar cells using a blend of quantum dots and nanorods. Adv. Funct. Mater. 2012, 22, 397–404. [Google Scholar] [CrossRef]
- Kang, Y.; Park, N.G.; Kim, D. Hybrid solar cells with vertically aligned CdTe nanorods and a conjugated polymer. Appl. Phys. Lett. 2005, 86, 1–3. [Google Scholar] [CrossRef]
- Chen, H.C.; Lai, C.W.; Wu, I.C.; Pan, H.R.; Chen, I.W.P.; Peng, Y.K.; Liu, C.L.; Chen, C.H.; Chou, P.T. Enhanced performance and air stability of 3.2% hybrid solar cells: How the functional polymer and CdTe nanostructure boost the solar cell efficiency. Adv. Mater. 2011, 23, 5451–5455. [Google Scholar] [CrossRef] [PubMed]
- Beek, W.J.E.; Wienk, M.M.; Kemerink, M.; Yang, X.; Janssen, R.A.J. Hybrid zinc oxide conjugated polymer bulk heterojunction solar cells. J. Phys. Chem. B 2005, 109, 9505–9516. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Chu, T.H.; Li, S.S.; Chuang, C.H.; Chang, C.H.; Su, W.F.; Chang, C.P.; Chu, M.W.; Chen, C.W. Interfacial nanostructuring on the performance of polymer/TiO2 nanorod bulk heterojunction solar cells. J. Am. Chem. Soc. 2009, 131, 3644–3649. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Kershaw, S.V.; Li, T.; Wang, C.; Zhang, X.; Wang, W.; Li, D.; Wang, Y.; Lu, M.; et al. Zn-alloyed CsPbI3 nanocrystals for highly efficient perovskite light-emitting devices. Nano Lett. 2019, 19, 1552–1559. [Google Scholar] [CrossRef]
- Mathias, F.; Tahir, M.N.; Tremel, W.; Zentel, R. Functionalization of TiO2 nanoparticles with semiconducting polymers containing a photocleavable anchor group and separation via irradiation afterward. Macromol. Chem. Phys. 2014, 215, 604–613. [Google Scholar] [CrossRef]
- Colvin, V.L.; Schlamp, M.C.; Alivisatos, A.P. Light-emitting diodes made from cadmium selenide nanocrystals and a semiconducting polymer. Nature 1994, 370, 354–357. [Google Scholar] [CrossRef]
- Reiss, P.; Couderc, E.; De Girolamo, J.; Pron, A. Conjugated polymers/semiconductor nanocrystals hybrid materials—Preparation, electrical transport properties and applications. Nanoscale 2011, 3, 446–489. [Google Scholar] [CrossRef]
- Chandrasekaran, J.; Nithyaprakash, D.; Ajjan, K.B.; Maruthamuthu, S.; Manoharan, D.; Kumar, S. Hybrid solar cell based on blending of organic and inorganic materials—An overview. Renew. Sustain. Energy Rev. 2011, 15, 1228–1238. [Google Scholar] [CrossRef]
- Zhao, L.; Lin, Z. Crafting semiconductor organic-inorganic nanocomposites via placing conjugated polymers in intimate contact with nanocrystals for hybrid solar cells. Adv. Mater. 2012, 24, 4353–4368. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.; Uddin, A. Organic-inorganic hybrid solar cells: A comparative review. Sol. Energy Mater. Sol. Cells 2012, 107, 87–111. [Google Scholar] [CrossRef]
- Liu, R. Hybrid organic/inorganic nanocomposites for photovoltaic cells. Materials 2014, 7, 2747–2771. [Google Scholar] [CrossRef] [PubMed]
- Arici, E.; Sariciftci, N.S.; Meissner, D. Hybrid solar cells based on nanoparticles of CuInS2 in organic matrices. Adv. Funct. Mater. 2003, 13, 165–170. [Google Scholar] [CrossRef]
- Olson, D.C.; Piris, J.; Collins, R.T.; Shaheen, S.E.; Ginley, D.S. Hybrid photovoltaic devices of polymer and Zno nanofiber composites. Thin Solid Film. 2006, 496, 26–29. [Google Scholar] [CrossRef]
- Tekin, E.; Smith, P.J.; Hoeppener, S.; Van Den Berg, A.M.J.; Susha, A.S.; Rogach, A.L.; Feldmann, J.; Schubert, U.S. InkJet printing of luminescent cdte nanocrystal-polymer composites. Adv. Funct. Mater. 2007, 17, 23–28. [Google Scholar] [CrossRef]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.W. Foldable transparent substrates with embedded electrodes for flexible electronics. ACS Appl. Mater. Interfaces 2015, 7, 18574–18580. [Google Scholar] [CrossRef]
- Liff, S.M.; Kumar, N.; McKinley, G.H. High-performance elastomeric nanocomposites via solvent-exchange processing. Nat. Mater. 2007, 6, 76–83. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, Y.; Sellinger, A.; Lu, M.; Huang, J.; Fan, H.; Haddad, R.; Lopez, G.; Burns, A.R.; Sasaki, D.Y.; et al. Self-assembly of mesoscopically ordered chromatic polydiacetylene/silica nanocomposites. Nature 2001, 410, 913–917. [Google Scholar] [CrossRef]
- Das, P.; Malho, J.-M.; Rahimi, K.; Schacher, F.H.; Wang, B.; Demco, D.E.; Walther, A. Nacre-mimetics with synthetic nanoclays up to ultrahigh aspect ratios. Nat. Commun. 2015, 6, 5967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.; Bhowmik, P.K. Wholly aromatic liquid-crystalline polyesters. Prog. Polym. Sci. 1997, 22, 1431–1502. [Google Scholar] [CrossRef]
- Davidson, P.; Gabriel, J.C.P. Mineral liquid crystals. Curr. Opin. Colloid Interface Sci. 2005, 9, 377–383. [Google Scholar] [CrossRef]
- Flory, P.J.; Ronca, G. Theory of systems of rodlike particles—1. Athermal systems. Mol. Cryst. Liq. Cryst. 1979, 54, 289–309. [Google Scholar] [CrossRef]
- Vlassopoulos, D. Determination of chain conformation of stiff polymers by depolarized rayleigh scattering in solution. Macromolecules 1996, 29, 8948–8953. [Google Scholar] [CrossRef]
- Zocher, H. Über freiwillige Struktur Bildung in Solen. (Eine neue Art anisotrop flüssiger Medien.). Z. für Anorg. Allg. Chem. 1925, 147, 91–110. [Google Scholar] [CrossRef]
- Wang, Y.; Takahashi, K.; Lee, K.; Cao, G. Nanostructured vanadium oxide electrodes for enhanced lithium-ion intercalation. Adv. Funct. Mater. 2006, 16, 1133–1144. [Google Scholar] [CrossRef]
- Wegner, S.; Börzsönyi, T.; Bien, T.; Rose, G.; Stannarius, R. Alignment and dynamics of elongated cylinders under shear. Soft Matter 2012, 8, 10950–10958. [Google Scholar] [CrossRef]
- Dessombz, A.; Chiche, D.; Davidson, P.; Panine, P.; Chanéac, C.; Jolivet, J.P. Design of liquid-crystalline aqueous suspensions of rutile nanorods: Evidence of anisotropic photocatalytic properties. J. Am. Chem. Soc. 2007, 129, 5904–5909. [Google Scholar] [CrossRef]
- Michot, L.J.; Bihannic, I.; Maddi, S.; Baravian, C.; Levitz, P.; Davidson, P. Sol/Gel and isotropic/nematic transitions in aqueous suspensions of natural nontronite clay. Influence of particle anisotropy. 1. Features of the I/N transition. Langmuir 2008, 24, 3127–3139. [Google Scholar] [CrossRef]
- Lou, X.; Daussin, R.; Cuenot, S.; Duwez, A.S.; Pagnoulle, C.; Detrembleur, C.; Bailly, C.; Jérôme, R. Synthesis of pyrene-containing polymers and noncovalent sidewall functionalization of multiwalled carbon nanotubes. Chem. Mater. 2004, 16, 4005–4011. [Google Scholar] [CrossRef]
- Bahun, G.J.; Wang, C.; Adronov, A. Solubilizing single-walled carbon nanotubes with pyrene-functionalized block copolymers. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1941–1951. [Google Scholar] [CrossRef]
- Meuer, S.; Braun, L.; Zentel, R. Solubilisation of multi walled carbon nanotubes by α-pyrene functionalised PMMA and their liquid crystalline self-organisation. Chem. Commun. 2008, 27, 3166. [Google Scholar] [CrossRef] [PubMed]
- Beek, W.J.E.; Wienk, M.M.; Janssen, R.A.J. Efficient hybrid solar cells from zinc oxide nanoparticles and a conjugated polymer. Adv. Mater. 2004, 16, 1009–1013. [Google Scholar] [CrossRef]
- Grätzel, M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef]
- Suri, P.; Mehra, R.M. Effect of electrolytes on the photovoltaic performance of a hybrid dye sensitized ZnO solar cell. Sol. Energy Mater. Sol. Cells 2007, 91, 518–524. [Google Scholar] [CrossRef]
- Sites, J.; Pan, J. Strategies to increase CdTe solar-cell voltage. Thin Solid Film. 2007, 515, 6099–6102. [Google Scholar] [CrossRef]
- Huynh, W.U.; Dittmer, J.J.; Alivisatos, A.P. Hybrid nanorod-polymer solar cells. Science 2002, 295, 2425–2428. [Google Scholar] [CrossRef] [Green Version]
- Zorn, M.; Zentel, R. Liquid crystalline orientation of semiconducting nanorods in a semiconducting matrix. Macromol. Rapid Commun. 2008, 29, 922–927. [Google Scholar] [CrossRef]
- Zorn, M.; Tahir, M.N.; Bergmann, B.; Tremel, W.; Grigoriadis, C.; Floudas, G.; Zentel, R. Orientation and dynamics of ZnO nanorod liquid crystals in electric fields. Macromol. Rapid Commun. 2010, 31, 1101–1107. [Google Scholar] [CrossRef]
- Zorn, M.; Weber, S.A.L.; Tahir, M.N.; Tremel, W.; Butt, H.J.; Berger, R.; Zentel, R. Light induced charging of polymer functionalized nanorods. Nano Lett. 2010, 10, 2812–2816. [Google Scholar] [CrossRef] [PubMed]
- Zur Borg, L.; Domanski, A.L.; Berger, R.; Zentel, R. Photoinduced Charge separation of self-organized semiconducting superstructures composed of a functional polymer-TiO2 hybrid. Macromol. Chem. Phys. 2013, 214, 975–984. [Google Scholar] [CrossRef]
- Zur, B.L.; Domanski, A.L.; Breivogel, A.; Bürger, M.; Berger, R.; Heinze, K.; Zentel, R. Light-induced charge separation in a donor–chromophore–acceptor nanocomposite Poly[TPA-Ru(Tpy)2]@ZnO. J. Mater. Chem. C 2013, 1, 1223–1230. [Google Scholar] [CrossRef]
- Oschmann, B.; Bresser, D.; Tahir, M.N.; Fischer, K.; Tremel, W.; Passerini, S.; Zentel, R. Polyacrylonitrile block copolymers for the preparation of a thin carbon coating around TiO2 nanorods for advanced lithium-ion batteries. Macromol. Rapid Commun. 2013, 34, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Oschmann, B.; Tahir, M.N.; Mueller, F.; Bresser, D.; Lieberwirth, I.; Tremel, W.; Passerini, S.; Zentel, R. Precursor polymers for the carbon coating of Au@ZnO multipods for application as active material in lithium-ion batteries. Macromol. Rapid Commun. 2015, 36, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.N.; Oschmann, B.; Buchholz, D.; Dou, X.; Lieberwirth, I.; Panthöfer, M.; Tremel, W.; Zentel, R.; Passerini, S. Extraordinary performance of carbon-coated anatase TiO2 as sodium-ion anode. Adv. Energy Mater. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Ji, W.; Jing, P.; Yuan, X.; Wang, Y.A.; Xiang, W.; Zhao, J. Efficient inverted quantum-dot light-emitting devices with TiO2/ZnO bilayer as the electron contact layer. Opt. Lett. 2014, 39, 426. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Z.; Jin, Y.; Niu, Y.; Cao, H.; Liang, X.; Chen, L.; Wang, J.; Peng, X. Solution-processed, high-performance light-emitting diodes based on quantum dots. Nature 2014, 515, 96–99. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Zhang, D.-D.; Wei, H.-X.; Ou, Q.-D.; Li, Y.-Q.; Tang, J.-X. Highly efficient quantum-dot light emitting diodes with sol-gel ZnO electron contact. Opt. Mater. Express 2017, 7, 2161. [Google Scholar] [CrossRef]
- Talapin, D.V.; Mekis, I.; Götzinger, S.; Kornowski, A.; Benson, O.; Weller, H. CdSe/CdS/ZnS and CdSe/ZnSe/ZnS core-shell-shell nanocrystals. J. Phys. Chem. B 2004, 108, 18826–18831. [Google Scholar] [CrossRef]
- Kwak, J.; Bae, W.K.; Lee, D.; Park, I.; Lim, J.; Park, M.; Cho, H.; Woo, H.; Yoon, D.Y.; Char, K.; et al. Bright and efficient full-color colloidal quantum dot light-emitting diodes using an inverted device structure. Nano Lett. 2012, 12, 2362–2366. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki, Y.; Supran, G.J.; Tisdale, W.A.; Bulović, V. Origin of efficiency roll-off in colloidal quantum-dot light-emitting diodes. Phys. Rev. Lett. 2013, 110, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Jeong, B.G.; Park, M.; Kim, J.K.; Pietryga, J.M.; Park, Y.S.; Klimov, V.I.; Lee, C.; Lee, D.C.; Bae, W.K. Influence of shell thickness on the performance of light-emitting devices based on CdSe/Zn1−xCdxS core/shell heterostructured quantum dots. Adv. Mater. 2014, 26, 8034–8040. [Google Scholar] [CrossRef]
- Kim, H.H.; Park, S.; Yi, Y.; Son, D.I.; Park, C.; Hwang, D.K.; Choi, W.K. Inverted quantum dot light emitting diodes using polyethylenimine ethoxylated modified ZnO. Sci. Rep. 2015, 5, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Cao, W.; Shewmon, N.T.; Yang, C.; Li, L.S.; Xue, J. High-efficiency, low turn-on voltage blue-violet quantum-dot-based light-emitting diodes. Nano Lett. 2015, 15, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Chen, J.; Zhao, D.; Huang, Q.; Khan, Q.; Liu, X.; Tao, Z.; Zhang, Z.; Lei, W. Surface plasmon-enhanced quantum dot light-emitting diodes by incorporating gold nanoparticles. Opt. Express 2016, 24, A33. [Google Scholar] [CrossRef]
- Jung, H.; Chung, W.; Lee, C.H.; Kim, S.H. Fabrication of white light-emitting diodes based on UV light-emitting diodes with conjugated polymers-(CdSe/ZnS) quantum dots as hybrid phosphors. J. Nanosci. Nanotechnol. 2012, 12, 5407–5411. [Google Scholar] [CrossRef]
- Park, J.S.; Han, J.; Ha, J.S.; Seong, T.Y. Polarity dependence of the electrical characteristics of Ag reflectors for high-power GaN-based light emitting diodes. Appl. Phys. Lett. 2014, 104, 1–5. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, W.C.; Wang, C.F.; Chen, S. In situ access to fluorescent dual-component polymers towards optoelectronic devices via inhomogeneous biphase frontal polymerization. RSC Adv. 2015, 5, 102294–102299. [Google Scholar] [CrossRef]
- Zorn, M.; Ki Bae, W.; Kwak, J.; Lee, H.; Lee, C.; Zentel, R.; Char, K. Quantum dot-block copolymer hybrids with improved properties and their application to quantum dot light-emitting devices. ACS Nano 2009, 3, 1063–1068. [Google Scholar] [CrossRef]
- Kwak, J.; Bae, W.K.; Zorn, M.; Woo, H.; Yoon, H.; Lim, J.; Kang, S.W.; Weber, S.; Butt, H.-J.; Zentel, R.; et al. Characterization of quantum dot/conducting polymer hybrid films and their application to light-emitting diodes. Adv. Mater. 2009, 21, 5022–5026. [Google Scholar] [CrossRef] [PubMed]
- Menk, F.; Fokina, A.; Oschmann, B.; Bauer, T.; Nyquist, Y.; Braun, L.; Kiehl, J.; Zentel, R. Functionalization of P3HT with various mono- and multidentate anchor groups. J. Braz. Chem. Soc. 2017. [Google Scholar] [CrossRef]
- zur Borg, L.; Lee, D.; Lim, J.; Bae, W.K.; Park, M.; Lee, S.; Lee, C.; Char, K.; Zentel, R. The effect of band gap alignment on the hole transport from semiconducting block copolymers to quantum dots. J. Mater. Chem. C 2013, 1, 1722. [Google Scholar] [CrossRef]
- Fokina, A.; Lee, Y.; Chang, J.H.; Braun, L.; Bae, W.K.; Char, K.; Lee, C.; Zentel, R. Side-chain conjugated polymers for use in the active layers of hybrid semiconducting polymer/quantum dot light emitting diodes. Polym. Chem. 2016, 7, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.D.; Kim, D.; Yoon, D.-E.; Lee, H.; Lim, J.; Bae, W.K.; Lee, D.C. Pushing the efficiency envelope for semiconductor nanocrystal-based electroluminescence devices using anisotropic nanocrystals. Chem. Mater. 2019, 31, 3066–3082. [Google Scholar] [CrossRef]
- Cheng, T.; Wang, Z.; Jin, S.; Wang, F.; Bai, Y.; Feng, H.; You, B.; Li, Y.; Hayat, T.; Tan, Z. Blue LEDs: Pure blue and highly luminescent quantum-dot light-emitting diodes with enhanced electron injection and exciton confinement via partially oxidized aluminum cathode (advanced optical materials 11/2017). Adv. Opt. Mater. 2017, 5. [Google Scholar] [CrossRef]
- Fu, Y.; Jiang, W.; Kim, D.; Lee, W.; Chae, H. Highly efficient and fully solution-processed inverted light-emitting diodes with charge control interlayers. ACS Appl. Mater. Interfaces 2018, 10, 17295–17300. [Google Scholar] [CrossRef]
- Khodabakhshi, E.; Klöckner, B.; Zentel, R.; Michels, J.J.; Blom, P.W.M. Suppression of electron trapping by quantum dot emitters using a grafted polystyrene shell. Mater. Horizons 2019, 6, 2024–2031. [Google Scholar] [CrossRef] [Green Version]















© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zentel, R. Polymer Coated Semiconducting Nanoparticles for Hybrid Materials. Inorganics 2020, 8, 20. https://doi.org/10.3390/inorganics8030020
Zentel R. Polymer Coated Semiconducting Nanoparticles for Hybrid Materials. Inorganics. 2020; 8(3):20. https://doi.org/10.3390/inorganics8030020
Chicago/Turabian StyleZentel, Rudolf. 2020. "Polymer Coated Semiconducting Nanoparticles for Hybrid Materials" Inorganics 8, no. 3: 20. https://doi.org/10.3390/inorganics8030020
APA StyleZentel, R. (2020). Polymer Coated Semiconducting Nanoparticles for Hybrid Materials. Inorganics, 8(3), 20. https://doi.org/10.3390/inorganics8030020