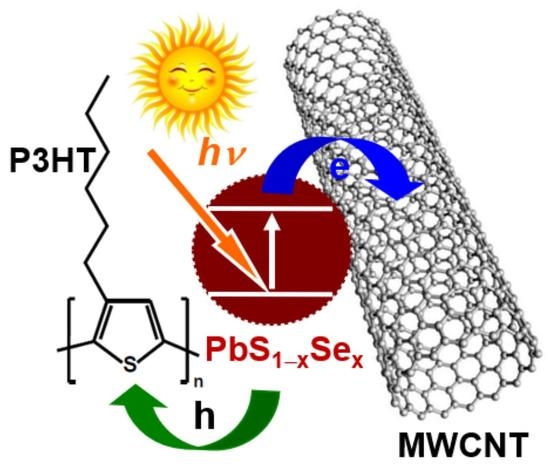
PbS1−xSex-Quantum-Dot@MWCNT/P3HT Nanocomposites with Tunable Photoelectric Conversion Performance
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Synthesis of PbS1−xSex Quantum Dots
3.2. Construction of PbS1−xSex-QD@MWCNT and PbS1−xSex-QD@MWCNT/P3HT
3.3. Structure, Morphology, and Optical Properties
3.4. Measurements of Photoelectrochemical and Photoelectric Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shrestha, A.; Batmunkh, M.; Tricoli, A.; Qiao, S.Z.; Dai, S. Near-Infrared Active Lead Chalcogenide Quantum Dots: Preparation, Post-Synthesis Ligand Exchange, and Applications in Solar Cells. Angew. Chem. Int. Ed. 2019, 58, 5202–5224. [Google Scholar] [CrossRef]
- Lu, H.; Carroll, G.M.; Neale, N.R.; Beard, M.C. Infrared Quantum Dots: Progress, Challenges, and Opportunities. ACS Nano 2019, 13, 939–953. [Google Scholar] [CrossRef]
- Tang, J.; Sargent, E.H. Infrared Colloidal Quantum Dots for Photovoltaics: Fundamentals and Recent Progress. Adv. Mater. 2011, 23, 12–29. [Google Scholar] [CrossRef]
- Hines, M.A.; Scholes, G.D. Colloidal PbS Nanocrystals with Size-Tunable Near-Infrared Emission: Observation of Post-Synthesis Self-Narrowing of the Particle Size Distribution. Adv. Mater. 2003, 15, 1844–1849. [Google Scholar] [CrossRef]
- Choi, M.J.; Pelayo Garcia de Arquer, F.; Proppe, A.H.; Seifitokaldani, A.; Choi, J.; Kim, J.; Baek, S.W.; Liu, M.; Sun, B.; Biondi, M.; et al. Cascade Surface Modification of Colloidal Quantum Dot Inks Enables Efficient Bulk Homojunction Photovoltaics. Nat. Commun. 2020, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Kelm, E.D.; Shukla, H.; Hinds, S.; Macneil, D.D.; Levina, L.; Sargent, E.H. Impact of Dithiol Treatment and Air Annealing on the Conductivity, Mobility, and Hole Density in PbS Colloidal Quantum Dot Solids. Appl. Phys. Lett. 2008, 92, 212105. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Xiong, K.; Wang, K.; Liang, G.; Li, M.; Tang, H.; Yang, K.; Huang, Z.; Lian, L.; Tan, M.; et al. Efficiently Passivated PbSe Quantum Dot Solids for Infrared Photovoltaics. ACS Nano 2021, 15, 3376–3386. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Luther, J.M.; Song, Q.; Hughes, B.K.; Perkins, C.L.; Nozik, A.J. Structural, Optical, and Electrical Properties of PbSe Nanocrystal Solids Treated Thermally or with Simple Amines. J. Am. Chem. Soc. 2008, 130, 5974–5985. [Google Scholar] [CrossRef]
- Luther, J.M.; Law, M.; Song, Q.; Perkins, C.L.; Beard, M.C.; Nozik, A.J. Structural, Optical, and Electrical Properties of Self-Assembled Films of PbSe Nanocrystals Treated with 1,2-Ethanedithiol. ACS Nano 2008, 2, 271–280. [Google Scholar] [CrossRef]
- Wise, F.W. Lead Salt Quantum Dots: The Limit of Strong Quantum Confinement. Acc. Chem. Res. 2000, 33, 773–780. [Google Scholar] [CrossRef]
- Kang, I.; Wise, F.W. Electronic Structure and Optical Properties of PbS and PbSe Quantum Dots. J. Opt. Soc. Am. B 1997, 14, 1632–1646. [Google Scholar] [CrossRef]
- Ellingson, R.J.; Beard, M.C.; Johnson, J.C.; Yu, P.; Micic, O.I.; Nozik, A.J.; Shabaev, A.; Efros, A.L. Highly Efficient Multiple Exciton Generation in Colloidal PbSe and PbS Quantum Dots. Nano Lett. 2005, 5, 865–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cademartiri, L.; Bertolotti, J.; Sapienza, R.; Wiersma, D.S.; Von Freymann, G.; Ozin, G.A. Multigram Scale, Solventless, and Diffusion-Controlled Route to Highly Monodisperse PbS Nanocrystals. J. Phys. Chem. B 2006, 110, 671–673. [Google Scholar] [CrossRef]
- Zhao, H.; Chaker, M.; Ma, D. Bimodal Photoluminescence during the Growth of PbS Quantum Dots. J. Phys. Chem. C 2009, 113, 6497–6504. [Google Scholar] [CrossRef]
- Konstantatos, G.; Howard, I.; Fischer, A.; Hoogland, S.; Clifford, J.; Klem, E.; Levina, L.; Sargent, E.H. Ultrasensitive Solution-Cast Quantum Dot Photodetectors. Nature 2006, 442, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, M.; Meyns, M.; Lesyuk, R.; Lehmann, H.; Klinke, C. Attachment of Colloidal Nanoparticles to Boron Nitride Nanotubes. Chem. Mater. 2017, 29, 726–734. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Baral, J.K.; Zhao, H.; Gonfa, B.A.; Truong, V.; Khakani, M.E.; Izquierdo, R.; Ma, D. Controlled Fabrication of PbS Quantum-Dot/Carbon-Nanotube Nanoarchitecture and its Significant Contribution to Near-Infrared Photon-to-Current Conversion. Adv. Funct. Mater. 2011, 21, 4010–4018. [Google Scholar] [CrossRef]
- Li, X.; Jia, Y.; Cao, A. Tailored Single-Walled Carbon Nanotube--CdS Nanoparticle Hybrids for Tunable Optoelectronic Devices. ACS Nano 2010, 4, 506–512. [Google Scholar] [CrossRef]
- Pei, Q.; Chen, Z.; Wang, S.; Zhang, D.; Ma, P.; Li, S.; Zhou, X.; Lin, Y. PbS Decorated Multi-Walled Carbon Nanotube/Ti Mesh Films as Efficient Counter Electrodes for Quantum Dots Sensitized Solar Cells. Sol. Energy 2019, 178, 108–113. [Google Scholar] [CrossRef]
- Jiang, X.; Schaller, R.D.; Lee, S.B.; Pietryga, J.M.; Klimov, V.I.; Zakhidov, A.A. PbSe Nanocrystal/Conducting Polymer Solar Cells with an Infrared Response to 2 Micron. J. Mater. Res. 2007, 22, 2204–2210. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Huang, S.; Patterson, R.; Halpert, J.E. Enhanced Mobility in PbS Quantum Dot Films via PbSe Quantum Dot Mixing for Optoelectronic Applications. J. Mater. Chem. C 2019, 7, 4497–4502. [Google Scholar] [CrossRef]
- Zhao, H.; Fan, Z.; Liang, H.; Selopal, G.S.; Gonfa, B.A.; Jin, L.; Soudi, A.; Cui, D.; Enrichi, F.; Natile, M.M.; et al. Controlling Photoinduced Electron Transfer from PbS@CdS Core@Shell Quantum Dots to Metal Oxide Nanostructured Thin Films. Nanoscale 2014, 6, 7004–7011. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.T.; Sunil Sharma, S.; Chen, S.A.; Komarov, P.V.; Ivanov, V.A.; Khokhlov, A.R. Polymer–Quantum Dot Composite Hybrid Solar Cells with a Bi-continuous Network Morphology Using the Block Copolymer Poly(3-hexylthiophene)-b-polystyrene or its Blend with Poly(3-hexylthiophene) as a Donor. Mater. Adv. 2021, 2, 1016–1023. [Google Scholar] [CrossRef]
- Kongkanand, A.; Tvrdy, K.; Takechi, K.; Kuno, M.; Kamat, P.V. Quantum Dot Solar Cells. Tuning Photoresponse through Size and Shape Control of CdSe-TiO2 Architecture. J. Am. Chem. Soc. 2008, 130, 4007–4015. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, H.; Wu, N.; Khakani, M.E.; Ma, D. Tuning the Charge-Transfer Property of PbS-Quantum Dot/TiO2-Nanobelt Nanohybrids via Quantum Confinement. J. Phys. Chem. Lett. 2010, 1, 1030–1035. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Yuan, J.; Wei, H.; Huang, X.; Han, L.; Wang, W.; Wang, H.; Ma, W. High-Efficiency Hybrid Solar Cells Based on Polymer/PbSxSe1−x Nanocrystals Benefiting from Vertical Phase Segregation. Adv. Mater. 2013, 25, 5772–5778. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, H.; Colbert, A.E.; Strein, E.; Janke, E.M.; Salvador, M.; Schlenker, C.W.; Ginger, D.S. Size-Dependent Charge Transfer Yields in Conjugated Polymer/Quantum Dot Blends. J. Phys. Chem. C 2014, 118, 5710–5715. [Google Scholar] [CrossRef]
- Akhtar, J.; Afzaal, M.; Banski, M.; Podhorodecki, A.; Syperek, M.; Misiewicz, J.; Bangert, U.; Hardman, S.O.; Graham, D.M.; Flavell, W.R.; et al. Controlled Synthesis of Tuned Bandgap Nanodimensional Alloys of PbSxSe1−x. J. Am. Chem. Soc. 2011, 133, 5602–5609. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.K.; Luther, J.M.; Semonin, O.E.; Nozik, A.J.; Beard, M.C. Tuning the Synthesis of Ternary Lead Chalcogenide Quantum Dots by Balancing Precursor Reactivity. ACS Nano 2011, 5, 183–190. [Google Scholar] [CrossRef]
- Gao, B.; Zhao, M.; Wang, Q.; Kang, K.; Xu, Z.; Zhang, H. Improved Synthesis of PbSxSe1−x Ternary Alloy Nanocrystals and their Nonlinear Optical Properties. New J. Chem. 2013, 37, 1692–1695. [Google Scholar] [CrossRef]
- Ren, J.; Ouyang, S.; Chen, H.; Umezawa, N.; Lu, D.; Wang, D.; Xu, H.; Ye, J. Effective Mineralization of Organic Dye under Visible-Light Irradiation over Electronic-Structure-Modulated Sn(Nb1−xTax)2O6 Solid Solutions. Appl. Catal. B Environ. 2015, 168, 243–249. [Google Scholar] [CrossRef]
- Wang, L.; Ouyang, S.; Ren, B.; Ye, J.; Wang, D. Enhanced Photocatalytic Degradation of 2-Propanol over Macroporous GaN/ZnO Solid Solution Prepared by a Novel Sol-Gel Method. APL Mater. 2015, 3, 104414. [Google Scholar] [CrossRef] [Green Version]
- Maeda, K.; Takata, T.; Hara, M.; Saito, N.; Inoue, Y.; Kobayashi, H.; Domen, K. GaN:ZnO Solid Solution as a Photocatalyst for Visible-Light-Driven Overall Water Splitting. J. Am. Chem. Soc. 2005, 127, 8286–8287. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Zhang, X.; Zhao, M.; Wang, X.; Ye, J.; Wang, D. Significant Enhancement in Photocatalytic Activity of (GaN)1−x(ZnO)x Nanowires via Solubility and Crystal Facet Tailoring. AIP Adv. 2018, 8, 015206. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Kako, T.; Ye, J. Efficient Photocatalytic Decomposition of Acetaldehyde over a Solid-Solution Perovskite (Ag0.75Sr0.25)(Nb0.75Ti0.25)O3 under Visible-Light Irradiation. J. Am. Chem. Soc. 2008, 130, 2724–2725. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Kako, T.; Ye, J. New Series of Solid-Solution Semiconductors (AgNbO3)1−x(SrTiO3)x with Modulated Band Structure and Enhanced Visible-Light Photocatalytic Activity. J. Phys. Chem. C 2009, 113, 3785–3792. [Google Scholar] [CrossRef]
- Ma, W.; Luther, J.M.; Zheng, H.; Yue, W.; Alivisatos, A.P. Photovoltaic Devices Employing Ternary PbSxSe1−x Nanocrystals. Nano Lett. 2009, 9, 1699–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, W.; Swisher, S.L.; Ewers, T.; Engel, J.; Ferry, V.E.; Atwater, H.A.; Alivisatos, A.P. Photovoltaic Performance of Ultrasmall PbSe Quantum Dots. ACS Nano 2011, 5, 8140–8147. [Google Scholar] [CrossRef] [Green Version]
- Murray, C.B.; Kagan, C.R.; Bawendi, M.G. Synthesis and Characterization of Monodisperse Nanocrystals and Close-Packed Nanocrystal Assemblies. Ann. Rev. Mater. Sci. 2000, 30, 545–610. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.; Kemp, K.W.; Thon, S.M.; Kim, J.Y.; Chou, K.W.; Amassian, A.; Sargent, E.H. High-Performance Quantum-Dot Solids via Elemental Sulfur Synthesis. Adv. Mater. 2014, 26, 3513–3519. [Google Scholar] [CrossRef] [PubMed]
- Weidman, M.C.; Beck, M.E.; Hoffman, R.S.; Prins, F.; Tisdale, W.A. Monodisperse, Air-Stable PbS Nanocrystals via Precursor Stoichiometry Control. ACS Nano 2014, 8, 6363–6371. [Google Scholar] [CrossRef] [Green Version]
- Klimov, V.I. Detailed-Balance Power Conversion Limits of Nanocrystal-Quantum-Dot Solar Cells in the Presence of Carrier Multiplication. Appl. Phys. Lett. 2006, 89, 123188. [Google Scholar] [CrossRef] [Green Version]
- Thomson, J.W.; Wang, X.; Hoch, L.; Faulkner, D.; Petrov, S.; Ozin, G.A. Discovery and Evaluation of a Single Source Selenium Sulfide Srecursor for the Synthesis of Alloy PbSxSe1−x Nanocrystals. J. Mater. Chem. 2012, 22, 5984–5989. [Google Scholar] [CrossRef]
- Yu, W.W.; Falkner, J.C.; Shih, B.S.; Colvin, V.L. Preparation and Characterization of Monodisperse PbSe Semiconductor Nanocrystals in a Noncoordinating Solvent. Chem. Mater. 2004, 16, 3318–3322. [Google Scholar] [CrossRef]
- Baranov, D.; Lynch, M.J.; Curtis, A.C.; Carollo, A.R.; Douglass, C.R.; Mateo-Tejada, A.M.; Jonas, D.M. Purification of Oleylamine for Materials Synthesis and Spectroscopic Diagnostics for trans Isomers. Chem. Mater. 2019, 31, 1223–1230. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Gutierrez, D.F.; Hernandez-Casillas, L.P.; Cappellari, M.V.; Fungo, F.; Martínez-Guerra, E.; García-Gutiérrez, D.I. Influence of the Capping Ligand on the Band Gap and Electronic Levels of PbS Nanoparticles through Surface Atomistic Arrangement Determination. ACS Omega 2018, 3, 393–405. [Google Scholar] [CrossRef]
- Landes, C.; Braun, M.; Burda, C.; El-Sayed, M.A. Observation of Large Changes in the Band Gap Absorption Energy of Small CdSe Nanoparticles Induced by the Adsorption of a Strong Hole Acceptor. Nano Lett. 2001, 1, 667–670. [Google Scholar] [CrossRef]
- Hyun, B.; Zhong, Y.; Bartnik, A.C.; Sun, L.; Abrun, H.D.; Wise, F.W.; Goodreau, J.D.; Matthews, J.R.; Leslie, T.D.; Borrelli, N.F. Electron Injection from Colloidal PbS Quantum Dots into Titanium Dioxide Nanoparticles. ACS Nano 2008, 2, 2206–2212. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pan, X.; Zheng, D.; Gao, Y.; Jiang, X.; Xu, M.; Chen, H. Hybrid Solar Cells Based on P3HT and Si@MWCNT Nanocomposite. Nanotechnology 2010, 21, 345201. [Google Scholar] [CrossRef]
- Solomeshch, O.; Kigel, A.; Saschiuk, A.; Medvedev, V.; Aharoni, A.; Razin, A.; Eichen, Y.; Banin, U.; Lifshitz, E.; Tessler, N. Optoelectronic Properties of Polymer-Nanocrystal Composites Active at Near-Infrared Wavelengths. J. Appl. Phys. 2005, 98, 074310. [Google Scholar] [CrossRef]
- Kucur, E.; Riegler, J.; Urban, G.A.; Nann, T. Determination of Quantum Confinement in CdSe Nanocrystals by Cyclic Voltammetry. J. Chem. Phys. 2003, 119, 2333–2337. [Google Scholar] [CrossRef]
- Pathak, P.; Podzorski, M.; Bahnemann, D.; Subramanian, V.R. One-Pot Fabrication of High Coverage PbS Quantum Dot Nanocrystal-Sensitized Titania Nanotubes for Photoelectrochemical Processes. J. Phys. Chem. C 2018, 122, 13659–13668. [Google Scholar] [CrossRef]
- Zhang, R.; Luo, Q.; Chen, H.; Yu, X.; Kuang, D.; Su, C. CdS/CdSe Quantum Qot Shell Decorated Vertical ZnO Nanowire Arrays by Spin-Coating-Based SILAR for Photoelectrochemical Cells and Quantum-Dot-Sensitized Solar cells. ChemPhysChem 2012, 13, 1435–1439. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Hu, H.; Ye, M.; Ye, J.; Wang, D. PbS1−xSex-Quantum-Dot@MWCNT/P3HT Nanocomposites with Tunable Photoelectric Conversion Performance. Inorganics 2021, 9, 87. https://doi.org/10.3390/inorganics9120087
Zhu H, Hu H, Ye M, Ye J, Wang D. PbS1−xSex-Quantum-Dot@MWCNT/P3HT Nanocomposites with Tunable Photoelectric Conversion Performance. Inorganics. 2021; 9(12):87. https://doi.org/10.3390/inorganics9120087
Chicago/Turabian StyleZhu, He, Huilin Hu, Minheng Ye, Jinhua Ye, and Defa Wang. 2021. "PbS1−xSex-Quantum-Dot@MWCNT/P3HT Nanocomposites with Tunable Photoelectric Conversion Performance" Inorganics 9, no. 12: 87. https://doi.org/10.3390/inorganics9120087
APA StyleZhu, H., Hu, H., Ye, M., Ye, J., & Wang, D. (2021). PbS1−xSex-Quantum-Dot@MWCNT/P3HT Nanocomposites with Tunable Photoelectric Conversion Performance. Inorganics, 9(12), 87. https://doi.org/10.3390/inorganics9120087