Early Response and Clinical Efficacy of a Mouthwash Containing Chlorhexidine, Anti Discoloration System, Polyvinylpyrrolidone/Vinyl Acetate and Sodium DNA in Periodontitis Model: A Triple-Blind Randomized Controlled Clinical Trial
Abstract
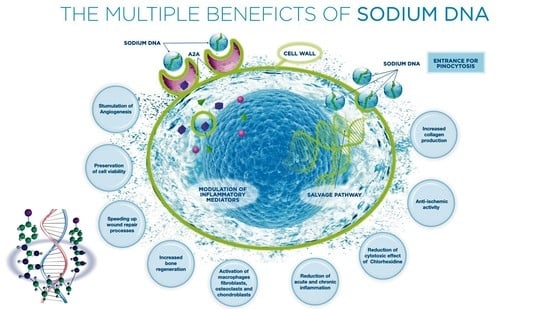
:1. Introduction
2. Materials and Methods
2.1. Trial Study Design
2.2. Mouthwash Solution Composition
2.3. Participants and Eligibility Criteria
- Patients with periodontitis with PD > 3 mm in more than or equal to 20 elements (Stage III) [22];
- Non-smokers or moderately smokers (<10 cigarettes/day);
- Patients with orthodontic devices;
- Intolerances or allergies to mouthwash;
- Smoking (>10 cigarettes/day) and consumption of alcohol;
- Patients undergoing radiant therapy/chemotherapy for less than 5 years;
- Immunosuppressed patients;
- Systemic, renal or cardiovascular diseases;
- Pregnant, breastfeeding women or patients undergoing antibiotic and anti-inflammatory therapy.
2.4. Allocation Procedure
2.5. Clinical Evaluation and Data Recording
2.6. Study Outcomes
- −
- FMPS: the presence/absence of plaque has been recorded in four sites per tooth and the percentage is calculated in relation to the surfaces. The results have been reported as percentage of the positive sites [23];
- −
- FMBS: the presence/absence of bleeding has been recorded at four sites per tooth and the percentage is calculated in relation to the surfaces. The results have been reported as percentage of the positive sites [23];
- −
- Gingival Index (Loe &Silness 1963): the health of periodontal tissues have been recorded according to the following criteria [24]:
- 0 = Normal gingiva;
- 1 = Mild inflammation and slight edema and color change in the absence of bleeding on probing;
- 2 = Moderate inflammation- reddened tissues, edematosis, and bleeding at the test;
- 3 = Severe inflammation marked redness, edema, ulceration, and tendency to bleeding.
2.7. Sample Size Calculation
2.8. Statistical Analysis
3. Results
3.1. Main Characteristics of the Study Population
3.2. Study Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHX | Chlorexidine |
| PVP/VA | Polyvinylpyrrolidone/vinyl acetate (PVP-VA) |
| ADS | Anti-discoloration System |
| FMPS | Full Mouth Plaque Score |
| FMBS | Full Mouth Bleeding Score |
| GI | Gingival Index |
| V1 | First Visit |
| V2 | Visit at the baseline |
| V3 | Visit after 2 weeks |
References
- Supranoto, S.; Slot, D.; Addy, M.; Van der Weijden, G. The Effect of Chlorhexidine Dentifrice or Gel versus Chlorhexidine Mouthwash on Plaque, Gingivitis, Bleeding and Tooth Discoloration: A Systematic Review. Int. J. Dent. Hyg. 2015, 13, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Slot, D.; Berchier, C.; Addy, M.; Van der Velden, U.; Van der Weijden, G. The Efficacy of Chlorhexidine Dentifrice or Gel on Plaque, Clinical Parameters of Gingival Inflammation and Tooth Discoloration: A Systematic Review. Int. J. Dent. Hyg. 2014, 12, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Murmura, G.; Vantaggiato, G.; Lauritano, D.; Silvestre-Rangil, J.; Di Cerbo, A.; Lorusso, F. Delayed Expansion of Atrophic Mandible (Deam): A Case Report. Oral Implantol. 2017, 10, 190–196. [Google Scholar] [CrossRef]
- Maglione, M.; Bevilacqua, L.; Dotto, F.; Costantinides, F.; Lorusso, F.; Scarano, A. Observational Study on the Preparation of the Implant Site with Piezosurgery vs. Drill: Comparison between the Two Methods in Terms of Postoperative Pain, Surgical Times, and Operational Advantages. BioMed Res. Int. 2019, 2019, 8483658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarano, A.; Inchingolo, F.; Lorusso, F. Environmental Disinfection of a Dental Clinic during the COVID-19 Pandemic: A Narrative Insight. BioMed Res. Int. 2020, 2020, 8896812. [Google Scholar] [CrossRef] [PubMed]
- Van Strydonck, D.A.C.; Slot, D.E.; Van der Velden, U.; Van der Weijden, F. Effect of a Chlorhexidine Mouthrinse on Plaque, Gingival Inflammation and Staining in Gingivitis Patients: A Systematic Review. J. Clin. Periodontol. 2012, 39, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Maddi, A.; Scannapieco, F.A. Oral Biofilms, Oral and Periodontal Infections, and Systemic Disease. Am. J. Dent. 2013, 26, 249–254. [Google Scholar]
- Pinto, G.; Silva, M.D.; Peddey, M.; Sillankorva, S.; Azeredo, J. The Role of Bacteriophages in Periodontal Health and Disease. Future Microbiol. 2016, 11, 1359–1369. [Google Scholar] [CrossRef]
- Torkzaban, P.; Ziaei, N.; Tootiaee, B.; Khoshhal, M.; Vafaee, F.; Panahandeh, N. Effect of Implant-Abutment Connection Type on Stress Distribution in Peri-Implant Bone and Abutment Micromovement: A Three-Dimensional Finite Element Analysis. J. Long-Term Eff. Med. Implant. 2019, 29, 113–124. [Google Scholar] [CrossRef]
- Ng, E.; Lim, L.P. An Overview of Different Interdental Cleaning Aids and Their Effectiveness. Dent. J. 2019, 7, E56. [Google Scholar] [CrossRef] [Green Version]
- Cortellini, P.; Labriola, A.; Zambelli, R.; Pini Prato, G.; Nieri, M.; Tonetti, M.S. Chlorhexidine with an Anti Discoloration System after Periodontal Flap Surgery: A Cross-over, Randomized, Triple-Blind Clinical Trial. J. Clin. Periodontol. 2008, 35, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.; Bruhn, G.; Heumann, C.; Netuschil, L.; Brecx, M.; Hoffmann, T. Effect of Two New Chlorhexidine Mouthrinses on the Development of Dental Plaque, Gingivitis, and Discolouration. A Randomized, Investigator-Blind, Placebo-Controlled, 3-Week Experimental Gingivitis Study. J. Clin. Periodontol. 2006, 33, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Amantea, M.; Tatullo, M. A Comparative, Randomized, Controlled Study on Clinical Efficacy and Dental Staining Reduction of a Mouthwash Containing Chlorhexidine 0.20% and Anti Discoloration System (ADS). Ann. Stomatol. 2015, 6, 35. [Google Scholar]
- Guerra, F.; Pasqualotto, D.; Rinaldo, F.; Mazur, M.; Corridore, D.; Nofroni, I.; Ottolenghi, L.; Nardi, G.M. Therapeutic Efficacy of Chlorhexidine-Based Mouthwashes and Its Adverse Events: Performance-Related Evaluation of Mouthwashes Added with Anti-Discoloration System and Cetylpyridinium Chloride. Int. J. Dent. Hyg. 2019, 17, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Cantore, S.; Farronato, D.; Cirulli, N.; Inchingolo, F.; Papa, F.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G.; Sardaro, N.; et al. Periodontal disease and bone pathogenesis: The crosstalk between cytokines and porphyromonas gingivalis. J. Biol. Regul. Homeost. Agents 2015, 29, 273–281. [Google Scholar]
- Inchingolo, F.; Martelli, F.S.; Gargiulo Isacco, C.; Borsani, E.; Cantore, S.; Corcioli, F.; Boddi, A.; Nguyễn, K.C.D.; De Vito, D.; Aityan, S.K.; et al. Chronic Periodontitis and Immunity, Towards the Implementation of a Personalized Medicine: A Translational Research on Gene Single Nucleotide Polymorphisms (SNPs) Linked to Chronic Oral Dysbiosis in 96 Caucasian Patients. Biomedicines 2020, 8, 115. [Google Scholar] [CrossRef]
- Rapone, B.; Corsalini, M.; Converti, I.; Loverro, M.T.; Gnoni, A.; Trerotoli, P.; Ferrara, E. Does Periodontal Inflammation Affect Type 1 Diabetes in Childhood and Adolescence? A Meta-Analysis. Front. Endocrinol. 2020, 11, 278. [Google Scholar] [CrossRef]
- Varoni, E.M.; Gargano, M.; Ludwig, N.; Lodi, G.; Sardella, A.; Carrassi, A. Efficacy of an Anti-Discoloration System (ADS) in a 0.12% Chlorhexidine Mouthwash: A Triple Blind, Randomized Clinical Trial. Am. J. Dent. 2017, 30, 235–242. [Google Scholar]
- Claydon, N.; Addy, M.; Jackson, R.; Smith, S.; Newcombe, R.G. Studies on the Effect of Polyvinyl Pyrrolidone on the Activity of Chlorhexidine Mouthrinses: Plaque and Stain: PVP and Chlorhexidine on Plaque and Stain. J. Clin. Periodontol. 2001, 28, 558–564. [Google Scholar] [CrossRef]
- Li, W.; Wang, R.E.; Finger, M.; Lang, N.P. Evaluation of the Antigingivitis Effect of a Chlorhexidine Mouthwash with or without an Antidiscoloration System Compared to Placebo during Experimental Gingivitis. J. Investig. Clin. Dent. 2014, 5, 15–22. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Vezzoli, E.; Conte, V.; Procacci, P.; Garcia-Godoy, F.; Brambilla, E. Effects of Na-DNA Mouthwash Solutions on Oral Soft Tissues. A Bioreactor-Based Reconstituted Human Oral Epithelium Model. Am. J. Dent. 2020, 33, 277–284. [Google Scholar] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A New Classification Scheme for Periodontal and Peri-Implant Diseases and Conditions-Introduction and Key Changes from the 1999 Classification. J Clin Periodontol 2018, 45 (Suppl. 20), S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Bentley, C.D.; Disney, J.A. A Comparison of Partial and Full Mouth Scoring of Plaque and Gingivitis in Oral Hygiene Studies. J. Clin. Periodontol. 1995, 22, 131–135. [Google Scholar] [CrossRef]
- Löe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef]
- Żukowski, P.; Maciejczyk, M.; Waszkiel, D. Sources of Free Radicals and Oxidative Stress in the Oral Cavity. Arch. Oral Biol. 2018, 92, 8–17. [Google Scholar] [CrossRef]
- Wang, X.; Shen, K.; Wang, J.; Liu, K.; Wu, G.; Li, Y.; Luo, L.; Zheng, Z.; Hu, D. Hypoxic Preconditioning Combined with Curcumin Promotes Cell Survival and Mitochondrial Quality of Bone Marrow Mesenchymal Stem Cells, and Accelerates Cutaneous Wound Healing via PGC-1α/SIRT3/HIF-1α Signaling. Free. Radic. Biol. Med. 2020, 159, 164–176. [Google Scholar] [CrossRef]
- Lindskog, S.; Blomlöf, L.; Hammarström, L. Repair of Periodontal Tissues in Vivo and in Vitro. J. Clin. Periodontol. 1983, 10, 188–205. [Google Scholar] [CrossRef]
- Scarano, A.; Noumbissi, S.; Gupta, S.; Inchingolo, F.; Stilla, P.; Lorusso, F. Scanning Electron Microscopy Analysis and Energy Dispersion X-Ray Microanalysis to Evaluate the Effects of Decontamination Chemicals and Heat Sterilization on Implant Surgical Drills: Zirconia vs. Steel. Appl. Sci. 2019, 9, 2837. [Google Scholar] [CrossRef] [Green Version]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Malcangi, G.; Xhajanka, E.; Scarano, A.; Lorusso, F.; Farronato, M.; Tartaglia, G.M.; Isacco, C.G.; et al. SARS-CoV-2 Disease Adjuvant Therapies and Supplements Breakthrough for the Infection Prevention. Microorganisms 2021, 9, 525. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Vezzoli, E.; Conte, V.; Sartori, P.; Procacci, P.; Brambilla, E. Activity of Experimental Mouthwashes and Gels Containing DNA-RNA and Bioactive Molecules against the Oxidative Stress of Oral Soft Tissues: The Importance of Formulations. A Bioreactor-Based Reconstituted Human Oral Epithelium Model. Molecules 2021, 26, 2976. [Google Scholar] [CrossRef]
- Thellung, S.; Florio, T.; Maragliano, A.; Cattarini, G.; Schettini, G. Polydeoxyribonucleotides Enhance the Proliferation of Human Skin Fibroblasts: Involvement of A2 Purinergic Receptor Subtypes. Life Sci. 1999, 64, 1661–1674. [Google Scholar] [CrossRef]
- Bültzingslöwen, I.V.; Brennan, M.T.; Spijkervet, F.K.; Logan, R.; Stringer, A.; Raber-Durlacher, J.E.; Keefe, D. Growth Factors and Cytokines in the Prevention and Treatment of Oral and Gastrointestinal Mucositis. Supportive Care Cancer 2006, 14, 519–527. [Google Scholar] [CrossRef]
- Ohsato, A.; Abe, M.; Ohkubo, K.; Yoshimasu, H.; Zong, L.; Hoshi, K.; Takato, T.; Yanagimoto, S.; Yamamoto, K. A Comparative Study of Oral Health Status between International and Japanese University Student Patients in Japan. Healthcare 2018, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Stadler, A.F.; Mendez, M.; Oppermann, R.V.; Gomes, S.C. Tooth Loss in Patients under Periodontal Maintenance in a Private Practice: A Retrospective Study. Braz. Dent. J. 2017, 28, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Rapone, B.; Ferrara, E.; Corsalini, M.; Qorri, E.; Converti, I.; Lorusso, F.; Delvecchio, M.; Gnoni, A.; Scacco, S.; Scarano, A. Inflammatory Status and Glycemic Control Level of Patients with Type 2 Diabetes and Periodontitis: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 3018. [Google Scholar] [CrossRef]
- Rapone, B.; Converti, I.; Santacroce, L.; Cesarano, F.; Vecchiet, F.; Cacchio, L.; Scacco, S.; Grassi, R.; Grassi, F.R.; Gnoni, A.; et al. Impact of Periodontal Inflammation on Nutrition and Inflammation Markers in Hemodialysis Patients. Antibiotics 2019, 8, E209. [Google Scholar] [CrossRef] [Green Version]
- Eberhard, J.; Jepsen, S.; Jervøe-Storm, P.-M.; Needleman, I.; Worthington, H.V. Full-Mouth Treatment Modalities (within 24 Hours) for Chronic Periodontitis in Adults. Cochrane Database Syst. Rev. 2015, 4, CD004622. [Google Scholar] [CrossRef] [Green Version]
- Rapone, B.; Ferrara, E.; Corsalini, M.; Converti, I.; Grassi, F.R.; Santacroce, L.; Topi, S.; Gnoni, A.; Scacco, S.; Scarano, A. The Effect of Gaseous Ozone Therapy in Conjunction with Periodontal Treatment on Glycated Hemoglobin Level in Subjects with Type 2 Diabetes Mellitus: An Unmasked Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 5467. [Google Scholar] [CrossRef]
- Di Venere, D.; Pettini, F.; Nardi, G.M.; Laforgia, A.; Stefanachi, G.; Notaro, V.; Rapone, B.; Grassi, F.R.; Corsalini, M. Correlation between Parodontal Indexes and Orthodontic Retainers: Prospective Study in a Group of 16 Patients. Oral Implantol. 2017, 10, 78. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Santacroce, L.; Cesarano, F.; Arazzi, M.; Di Liberato, L.; Scacco, S.; Grassi, R.; Grassi, F.R.; Gnoni, A. Periodontal Microbiological Status Influences the Occurrence of Cyclosporine-A and Tacrolimus-Induced Gingival Overgrowth. Antibiotics 2019, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- Hashim, D.; Cionca, N.; Combescure, C.; Mombelli, A. The Diagnosis of Peri-implantitis: A Systematic Review on the Predictive Value of Bleeding on Probing. Clin. Oral Implant. Res. 2018, 29, 276–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halai, H.; Somani, C.; Donos, N.; Nibali, L. Periodontal Status of Children with Primary Immunodeficiencies: A Systematic Review. Clin. Oral Investig. 2020, 24, 1939–1951. [Google Scholar] [CrossRef] [Green Version]
- Grassi, F.R.; Grassi, R.; Rapone, B.; Alemanno, G.; Balena, A.; Kalemaj, Z. Dimensional Changes of Buccal Bone Plate in Immediate Implants Inserted through Open Flap, Open Flap and Bone Grafting and Flapless Techniques: A Cone-Beam Computed Tomography Randomized Controlled Clinical Trial. Clin. Oral Implant. Res. 2019, 30, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.; Duane, B. Evidence That Full-Mouth Scaling Superior to Conventional Treatment Approaches Is Unclear: Question: Should Adults with Chronic Periodontitis Have Their Treatment Carried out within a 24-Hour Period? Evid.-Based Dent. 2016, 17, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Steffen, P.; Muller-Campanile, V.; Suvan, J.; Lang, N.P. Initial Extractions and Tooth Loss during Supportive Care in a Periodontal Population Seeking Comprehensive Care: Extractions in Periodontal Patients. J. Clin. Periodontol. 2000, 27, 824–831. [Google Scholar] [CrossRef] [Green Version]
- König, J.; Plagmann, H.-C.; Rühling, A.; Kocher, T. Tooth Loss and Pocket Probing Depths in Compliant Periodontally Treated Patients: A Retrospective Analysis: Tooth Loss and Pocket Probing Depths. J. Clin. Periodontol. 2002, 29, 1092–1100. [Google Scholar] [CrossRef]
- Carnevale, G.; Cairo, F.; Tonetti, M.S. Long-Term Effects of Supportive Therapy in Periodontal Patients Treated with Fibre Retention Osseous Resective Surgery. II: Tooth Extractions during Active and Supportive Therapy. J. Clin. Periodontol. 2007, 34, 342–348. [Google Scholar] [CrossRef]
- Matthews, D.C.; Smith, C.G.; Hanscom, S.L. Tooth Loss in Periodontal Patients. J.-Can. Dent. Assoc. 2001, 67, 207–210. [Google Scholar]
- Corsalini, M.; Di Venere, D.; Sportelli, P.; Magazzino, D.; Ripa, C.; Cantatore, F.; Cagnetta, G.; De Rinaldis, C.; Montemurro, N.; De Giacomo, A. Evaluation of prosthetic quality and masticatory efficiency in patients with total removable prosthesis: Study of 12 cases. Oral Implantol. 2018, 11, 230–240. [Google Scholar]
- Quaglia, E.; Moscufo, L.; Corsalini, M.; Coscia, D.; Sportelli, P.; Cantatore, F.; De Rinaldis, C.; Rapone, B.; Carossa, M.; Carossa, S. Polyamide vs. Silk Sutures in the Healing of Postextraction Sockets: A Split Mouth Study. Oral Implantol. 2018, 11, 115–120. [Google Scholar]
- Scarano, A.; Inchingolo, F.; Rapone, B.; Festa, F.; Tari, S.R.; Lorusso, F. Protective Face Masks: Effect on the Oxygenation and Heart Rate Status of Oral Surgeons during Surgery. Int. J. Environ. Res. Public Health 2021, 18, 2363. [Google Scholar] [CrossRef]
- Lorusso, F.; Noumbissi, S.; Francesco, I.; Rapone, B.; Khater, A.G.A.; Scarano, A. Scientific Trends in Clinical Research on Zirconia Dental Implants: A Bibliometric Review. Materials 2020, 13, 5534. [Google Scholar] [CrossRef]
- Corsalini, M.; Di Venere, D.; Carossa, M.; Ripa, M.; Sportelli, P.; Cantatore, F.; De Rinaldis, C.; Di Santantonio, G.; Lenoci, G.; Barile, G. Comparative clinical study between zirconium-ceramic and metal-ceramic fixed rehabilitations. Oral Implantol. 2018, 11, 150–160. [Google Scholar]
- Solderer, A.; Kaufmann, M.; Hofer, D.; Wiedemeier, D.; Attin, T.; Schmidlin, P.R. Efficacy of Chlorhexidine Rinses after Periodontal or Implant Surgery: A Systematic Review. Clin. Oral Investig. 2019, 23, 21–32. [Google Scholar] [CrossRef] [Green Version]
- da Costa, L.F.N.P.; da Silva Furtado Amaral, C.; da Silva Barbirato, D.; Leão, A.T.T.; Fogacci, M.F. Chlorhexidine Mouthwash as an Adjunct to Mechanical Therapy in Chronic Periodontitis: A Meta-Analysis. J. Am. Dent. Assoc. 2017, 148, 308–318. [Google Scholar] [CrossRef]
- Richards, D. Chlorhexidine Mouthwash Plaque Levels and Gingival Health. Evid.-Based Dent. 2017, 18, 37–38. [Google Scholar] [CrossRef]
- da Silveira Teixeira, D.; de Figueiredo, M.A.Z.; Cherubini, K.; de Oliveira, S.D.; Salum, F.G. The Topical Effect of Chlorhexidine and Povidone-Iodine in the Repair of Oral Wounds. A Review. Stomatologija 2019, 21, 35–41. [Google Scholar] [PubMed]
- Eggers, M.; Koburger-Janssen, T.; Eickmann, M.; Zorn, J. In Vitro Bactericidal and Virucidal Efficacy of Povidone-Iodine Gargle/Mouthwash against Respiratory and Oral Tract Pathogens. Infect. Dis. Ther. 2018, 7, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Düzkaya, D.S.; Uysal, G.; Bozkurt, G.; Yakut, T.; Çitak, A. Povidone-Iodine, 0.05% Chlorhexidine Gluconate, or Water for Periurethral Cleaning Before Indwelling Urinary Catheterization in a Pediatric Intensive Care: A Randomized Controlled Trial. J. Wound Ostomy Cont. Nurs. 2017, 44, 84–88. [Google Scholar] [CrossRef]
- Sharma, S.; Saimbi, C.S.; Koirala, B.; Shukla, R. Effect of Various Mouthwashes on the Levels of Interleukin-2 and Interferon-Gamma in Chronic Gingivitis. J. Clin. Pediatr. Dent. 2008, 32, 111–114. [Google Scholar] [CrossRef]




| Pocket Depth Grade | Test Group- Baseline | Test Group-Post Treatment (2 Weeks) | Control Group- Baseline | Control Group- (2 Weeks) |
|---|---|---|---|---|
| 1–2 mm | 29.70% | 32.50% | 37.30% | 37.10% |
| 3–4 mm | 44.30% | 41.60% | 31.90% | 32.40% |
| 5–6 mm | 21.70% | 21.50% | 27.40% | 27.20% |
| >6 mm | 4.30% | 4.40% | 3.40% | 3.30% |
| FMPS (Mean, SD) | Test Group | Control Group | p Value |
|---|---|---|---|
| Baseline | 52.7% ± 9.2% [95%CI: −64.20/169.6] | 58.2% ± 6.1% [95%CI: −19.31/135.7] | p = 0.517 |
| 1 week | 13.3% ± 5.6% [95%CI:−57.85/84.45] | 18.7% ± 4.3% [95%CI: −35.94/73.34] | p = 0.031 |
| 2 weeks | 14.2% ± 4.1% [95%CI: −37.90/66.30] | 20.3% ± 5.2% [95%CI: −45.77/86.37] | p = 0.046 |
| FMBS (mean, SD) | Test Group | Control Group | p value |
| Baseline | 46.7% ± 8.7% [95%CI: −63.84/157.2] | 49.2% ± 6.2% [95%CI: −29.58/128.0] | p = 0.731 |
| 1 week | 12.7% ± 4.2% [95%CI: −40.67/66.07] | 18.5% ± 5.9% [95%CI: −56.47/93.47] | p = 0.026 |
| 2 weeks | 13.1% ± 3.2% [95%CI: −27.56/53.66] | 19.8% ± 4.9% [95%CI: −42.46/82.06] | p = 0.041 |
| Gingival Index (mean, SD) | Test Group | Control Group | p value |
| Baseline | 2.75% ± 0.17% [95%CI: −3.122/8.822] | 2.71% ± 0.11% [95%CI: −3.770/9.190] | p = 0.937 |
| 1 week | 1.14% ± 0.55% [95%CI: −5.848/8.128] | 1.75% ± 0.49% [95%CI: −4.476/7.976] | p = 0.046 |
| 2 weeks | 1.09% ± 0.44% [95%CI: −4.501/6.681] | 1.96% ± 0.39% [95%CI: −2.995/6.915] | p = 0.038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorusso, F.; Tartaglia, G.; Inchingolo, F.; Scarano, A. Early Response and Clinical Efficacy of a Mouthwash Containing Chlorhexidine, Anti Discoloration System, Polyvinylpyrrolidone/Vinyl Acetate and Sodium DNA in Periodontitis Model: A Triple-Blind Randomized Controlled Clinical Trial. Dent. J. 2022, 10, 101. https://doi.org/10.3390/dj10060101
Lorusso F, Tartaglia G, Inchingolo F, Scarano A. Early Response and Clinical Efficacy of a Mouthwash Containing Chlorhexidine, Anti Discoloration System, Polyvinylpyrrolidone/Vinyl Acetate and Sodium DNA in Periodontitis Model: A Triple-Blind Randomized Controlled Clinical Trial. Dentistry Journal. 2022; 10(6):101. https://doi.org/10.3390/dj10060101
Chicago/Turabian StyleLorusso, Felice, Gianluca Tartaglia, Francesco Inchingolo, and Antonio Scarano. 2022. "Early Response and Clinical Efficacy of a Mouthwash Containing Chlorhexidine, Anti Discoloration System, Polyvinylpyrrolidone/Vinyl Acetate and Sodium DNA in Periodontitis Model: A Triple-Blind Randomized Controlled Clinical Trial" Dentistry Journal 10, no. 6: 101. https://doi.org/10.3390/dj10060101
APA StyleLorusso, F., Tartaglia, G., Inchingolo, F., & Scarano, A. (2022). Early Response and Clinical Efficacy of a Mouthwash Containing Chlorhexidine, Anti Discoloration System, Polyvinylpyrrolidone/Vinyl Acetate and Sodium DNA in Periodontitis Model: A Triple-Blind Randomized Controlled Clinical Trial. Dentistry Journal, 10(6), 101. https://doi.org/10.3390/dj10060101