The Correlation of Swedish Snus, Nicotine Pouches and Other Tobacco Products with Oral Mucosal Health and Salivary Biomarkers
Abstract
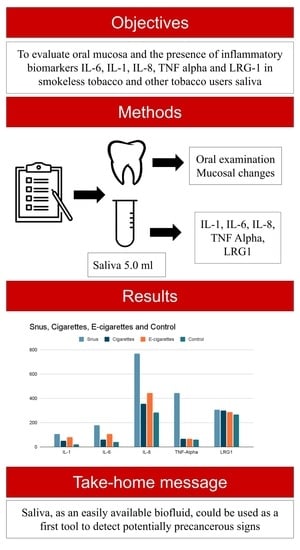
:1. Introduction
2. Materials and Methods
2.1. Research Ethics
2.2. Data Collection and Research Design
2.3. Oral Examination
2.4. Measured Variables
2.4.1. Inflammatory Biomarkers (IL-1, IL-6, IL-8 and TNF Alpha) Detection
2.4.2. LRG1 Detection
2.5. Statistical Analysis
3. Results
3.1. Results of Tobacco and Control Groups
3.2. Results of Mucosal Changes
3.3. Results of Inflammatory Biomarkers and LRG1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2017: Monitoring Tobacco Use and Prevention Policies; World Health Organization: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/handle/10665/255874 (accessed on 20 March 2022).
- Clarke, E.; Thompson, K.; Weaver, S.; Thompson, J.; O’Connell, G. Snus: A compelling harm reduction alternative to cigarettes. Harm Reduct. J. 2019, 16, 62. [Google Scholar] [CrossRef]
- European Tobacco Use. Trends Report 2019. World Health Organisation. Available online: https://www.euro.who.int/__data/assets/pdf_file/0009/402777/Tobacco-Trends-Report-ENG-WEB.pdf (accessed on 20 March 2022).
- Salari, N.; Hosseinian-Far, A.; Jalali, R.; Vaisi-Raygani, A.; Rasoulpoor, S.; Mohammadi, M.; Rasoulpoor, S.; Khaledi-Paveh, B. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: A systematic review and meta-analysis. Glob. Health 2020, 16, 57. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Sciomer, S.; Cocchi, C.; Maffei, S.; Gallina, S. Quarantine during COVID-19 outbreak: Changes in diet and physical activity increase the risk of cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1409–1417. [Google Scholar] [CrossRef]
- Mündel, T. Nicotine: Sporting Friend or Foe? A Review of Athlete Use, Performance Consequences and Other Considerations. Sports Med. 2017, 47, 2497–2506. [Google Scholar] [CrossRef]
- Zandonai, T.; Chiamulera, C.; Mancabelli, A.; Falconieri, D.; Diana, M. A Preliminary Investigation on Smokeless Tobacco Use and Its Cognitive Effects Among Athletes. Front. Pharmacol. 2018, 9, 216. [Google Scholar] [CrossRef]
- World Anti-Doping Agency, the 2021 Monitoring Program, Montreal. Available online: https://www.wada-ama.org/sites/default/files/resources/files/2021list_monitoring_program_en_0.pdf (accessed on 20 May 2022).
- Chaffee, B.W.; Couch, E.T.; Walsh, M.M. Smokeless Tobacco in Sport and Use Among Adolescents. UCSF: Center for Tobacco Control Research and Education 2015. Available online: https://escholarship.org/uc/item/6rc6v9t2 (accessed on 20 March 2022).
- Vogel, E.A.; Barrington-Trimis, J.L.; Kechter, A.; Tackett, A.P.; Liu, F.; Sussman, S.; Lerman, C.; Unger, J.B.; Hughes Halbert, C.; Chaffee, B.W.; et al. Differences in Young Adults’ Perceptions of and Willingness to Use Nicotine Pouches by Tobacco Use Status. Int. J. Environ. Res. Public Health 2022, 19, 2685. [Google Scholar] [CrossRef]
- Müller, S. Frictional Keratosis, Contact Keratosis and Smokeless Tobacco Keratosis: Features of Reactive White Lesions of the Oral Mucosa. Head Neck Pathol. 2019, 13, 16–24. [Google Scholar] [CrossRef]
- Greer, R.O. Oral Manifestations of Smokeless Tobacco Use. Otolaryngol. Clin. N. Am. 2011, 44, 31–56. [Google Scholar] [CrossRef]
- Mehrotra, R.; Yadav, A.; Sinha, D.N.; Parascandola, M.; John, R.M.; Ayo-Yusuf, O.; Nargis, N.; Hatsukami, D.K.; Warnakulasuriya, S.; Straif, K.; et al. Smokeless tobacco control in 180 countries across the globe: Call to action for full implementation of WHO FCTC measures. Lancet Oncol. 2019, 20, e208–e217. [Google Scholar] [CrossRef]
- Španko, M.; Strnadová, K.; Pavlíček, A.J.; Szabo, P.; Kodet, O.; Valach, J.; Dvořánková, B.; Smetana, K., Jr.; Lacina, L. IL-6 in the Ecosystem of Head and Neck Cancer: Possible Therapeutic Perspectives. Int. J. Mol. Sci. 2021, 22, 11027. [Google Scholar] [CrossRef]
- Ferrari, E.; Pezzi, M.E.; Cassi, D.; Pertinhez, T.A.; Spisni, A.; Meleti, M. Salivary Cytokines as Biomarkers for Oral Squamous Cell Carcinoma: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 6795. [Google Scholar] [CrossRef]
- Chiamulera, M.M.A.; Zancan, C.B.; Remor, A.P.; Cordeiro, M.F.; Gleber-Netto, F.O.; Baptistella, A.R. Salivary cytokines as biomarkers of oral cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 205. [Google Scholar] [CrossRef]
- Sharma, M.; Bairy, I.; Pai, K.; Satyamoorthy, K.; Prasad, S.; Berkovitz, B.; Radhakrishnan, R. Salivary IL-6 levels in oral leukoplakia with dysplasia and its clinical relevance to tobacco habits and periodontitis. Clin. Oral Investig. 2010, 15, 705–714. [Google Scholar] [CrossRef]
- Babiuch, K.; Kuśnierz-Cabala, B.; Kęsek, B.; Okoń, K.; Darczuk, D.; Chomyszyn-Gajewska, M. Evaluation of Proinflammatory, NF-kappaB Dependent Cytokines: IL-1α, IL-6, IL-8, and TNF-α in Tissue Specimens and Saliva of Patients with Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders. J. Clin. Med. 2020, 9, 867. [Google Scholar] [CrossRef]
- Chang, S.C.; Lin, W.L.; Chang, Y.F.; Lee, C.T.; Wu, J.S.; Hsu, P.H.; Chang, C.F. Glycoproteomic identification of novel plasma biomarkers for oral cancer. J. Food Drug Anal. 2019, 27, 483–493. [Google Scholar] [CrossRef]
- Khan, S.Z.; Farooq, A.; Masood, M.; Shahid, A.; Khan, I.U.; Nisar, H.; Fatima, I. Smokeless tobacco use and risk of oral cavity cancer. Turk. J. Med. Sci. 2020, 50, 291–297. [Google Scholar] [CrossRef]
- Gupta, B.; Johnson, N.W. Systematic review and meta-analysis of association of smokeless tobacco and of betel quid without tobacco with incidence of oral cancer in South Asia and the Pacific. PLoS ONE 2014, 9, e113385. [Google Scholar] [CrossRef]
- Donald, P.M.; Renjith, G.; Arora, A. Tobacco Pouch Keratosis in a young individual: A brief description. J. Indian Soc. Periodontol. 2017, 21, 249–251. [Google Scholar] [CrossRef]
- Mu, G.; Wang, J.; Liu, Z.; Zhang, H.; Zhou, S.; Xiang, Q.; Cui, Y. Association between smokeless tobacco use and oral cavity cancer risk in women compared with men: A systematic review and meta-analysis. BMC Cancer 2021, 21, 960. [Google Scholar] [CrossRef]
- Smyth, E.M.; Kulkarni, P.; Claye, E.; Stanfill, S.; Tyx, R.; Maddox, C.; Mongodin, E.F.; Sapkota, A.R. Smokeless tobacco products harbor diverse bacterial microbiota that differ across products and brands. Appl. Microbiol. Biotechnol. 2017, 101, 5391–5403. [Google Scholar] [CrossRef]
- Fadus, M.C.; Smith, T.T.; Squeglia, L.M. The rise of e-cigarettes, pod mod devices, and JUUL among youth: Factors influencing use, health implications, and downstream effects. Drug Alcohol Depend. 2019, 201, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC); Centre for Disease Prevention and Control (Latvia); World Health Organization (WHO). Latvia Global Youth Tobacco Survey 2019; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA; Available online: https://cdn.who.int/media/docs/default-source/ncds/ncd-surveillance/data-reporting/latvia/latvia-gyts-2019-factsheet-(ages-13-15)-final_508tagged.pdf?sfvrsn=74ca0219_1&download=true (accessed on 30 May 2022).
- Centers for Disease Control and Prevention (CDC); Centre for Disease Prevention and Control (Latvia); World Health Organization (WHO). Latvia Global Youth Tobacco Survey 2014; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA; Available online: https://extranet.who.int/ncdsmicrodata/index.php/catalog/505 (accessed on 30 May 2022).
- Sahibzada, H.A.; Khurshid, Z.; Khan, R.S.; Naseem, M.; Siddique, K.M.; Mali, M.; Zafar, M.S. Salivary IL-8, IL-6 and TNF-α as Potential Diagnostic Biomarkers for Oral Cancer. Diagnostics 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Jacobs, R. Proinflammatory cytokine levels in oral lichen planus, oral leukoplakia, and oral submucous fibrosis. J. Korean Assoc. Oral Maxillofac. Surg. 2015, 41, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T.; Vivar, J.C.; Tam, J.; Hammad, H.T.; Christensen, C.H.; van Bemmel, D.M.; Das, B.; Danilenko, U.; Chang, C.M. Biomarkers of Potential Harm among Adult Cigarette and Smokeless Tobacco Users in the PATH Study Wave 1 (2013–2014): A Cross-sectional Analysis. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Nijakowski, K.; Gruszczyński, D.; Kopała, D.; Surdacka, A. Salivary Metabolomics for Oral Squamous Cell Carcinoma Diagnosis: A Systematic Review. Metabolites 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, R.; Bollinger, J.G.; Rivera, C.; Ribeiro, A.C.; Brandão, T.B.; Paes Leme, A.F.; MacCoss, M.J. A targeted proteomic strategy for the measurement of oral cancer candidate biomarkers in human saliva. Proteomics 2016, 16, 159–173. [Google Scholar] [CrossRef]
- Lunell, E.; Fagerström, K.; Hughes, J.; Pendrill, R. Pharmacokinetic Comparison of a Novel Non-tobacco-Based Nicotine Pouch (ZYN) With Conventional, Tobacco-Based Swedish Snus and American Moist Snuff. Nicotine Tob Res. 2020, 22, 1757–1763. [Google Scholar] [CrossRef]
- Azzopardi, D.; Liu, C.; Murphy, J. Chemical characterization of tobacco-free “modern” oral nicotine pouches and their position on the toxicant and risk continuums. Drug Chem. Toxicol. 2021, 1–9. [Google Scholar] [CrossRef]




| Group | All (n = 76) | Women (n = 38) | Men (n = 38) |
|---|---|---|---|
| Age | 24.46 | 23.71 | 25.21 |
| Snus (%) | 15.8 | 16.7 | 83.3 |
| Cigarettes (%) | 25 | 47.4 | 52.6 |
| E-cigarettes (%) | 10.5 | 62.5 | 37.5 |
| Control group (%) | 48.7 | 59.5 | 40.5 |
| Research Group | Sex, n | Average Age, Years | Tobacco Product Type |
|---|---|---|---|
| Snus | Women n = 2 Men n = 10 | 25.08 | Swedish snus, nicotine sachets |
| Cigarettes | Women n = 9 Men n = 10 | 24.74 | cigarettes |
| E-cigarettes | Women n = 5 Men n = 3 | 23.00 | e-cigarettes |
| Control | Women n = 22 Men n = 15 | 24.43 | - |
| Total | Women n = 38 Men n = 38 | 24.46 | - |
| Association with mucosal changes | Women p < 0.05 Men p > 0.05 (Phi 0.285) * | p > 0.05 ** | p < 0.05 (0.417) *** |
| Tobacco Type Used | Tobacco Units per Day Used | Duration of Tobacco Product Used, Years | Characteristics of Mucosa | Location |
|---|---|---|---|---|
| Snus | 5–10 | 2–5 | White, leathery lesion (Figure 1) | Above tooth nr. 13 to tooth nr. 22 |
| Snus | 5–10 | 2–5 | White round lesion (Figure 2) | Above tooth nr. 13, 12 |
| Snus | 5–10 | 2–5 | White, grainy lesion (Figure 3) | Above tooth nr. 13/12 and 22/23 |
| Snus | 5–10 | 5–10 | White lesion | Above left and write upper premolars |
| Snus | 5–10 | 5–10 | White, linear lesions (Figure 4) | Right upper vestibule |
| Snus | 5–10 | 15 | White leathery lesions | Above upper central incisors |
| Snus | 10–20 | 5–10 | White, localized lesions | Above left lateral incisor |
| Snus | 5–10 | 2–5 | White, round localized lesions | Above left and right upper canines |
| Snus | 5–10 | 2–5 | White lesion | Above upper left and right premolars |
| Group | IL-1 (pg/mL) | IL-6 (pg/mL) | IL-8 (pg/mL) | TNF-Alpha (pg/mL) | LRG1 (mg/mL) |
|---|---|---|---|---|---|
| Snus | 105.14 (69.50) | 177.43 (50.34) | 767.43 (254.98) | 445.08 (122.71) | 306.35 (293.78) |
| Cigarettes | 49.69 (82.17) | 59.00 (94.59) | 355.52 (120.67) | 66.59 (92.44) | 300.76 (170.90) |
| E-cigarettes | 78.71 (92.37) | 107.39 (70.92) | 445.09 (135.12) | 66.60 (181.63) | 285.78 (130.46) |
| Control | 21.32 (26.53) | 40.54 (78.34) | 281.85 (249.13) | 60.22 (69.59) | 265.53 (90.98) |
| IL-1 (pg/mL) | IL-6 (pg/mL) | IL-8 (pg/mL) | TNF-Alpha (pg/mL) | LRG1 (mg/mL) | |
|---|---|---|---|---|---|
| Correlation with mucosal changes | 0.071 p > 0.05 | 0.344 p < 0.05 | 0.164 p > 0.05 | 0.143 p > 0.05 | −0.105 p > 0.05 |
| Correlation with tobacco product | 0.039 p > 0.05 | 0.260 p < 0.05 | 0.01 p > 0.05 | −0.102 p > 0.05 | −0.052 p > 0.05 |
| Correlation with duration of tobacco consumption | 0.102 p > 0.05 | 0.191 p > 0.05 | 0.081 p > 0.05 | −0.029 p > 0.05 | 0 p > 0.05 |
| Correlation with tobacco units used per day | 0.024 p > 0.05 | 0.144 p > 0.05 | 0.017 p > 0.05 | −0.095 p > 0.05 | −0.084 p > 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miluna, S.; Melderis, R.; Briuka, L.; Skadins, I.; Broks, R.; Kroica, J.; Rostoka, D. The Correlation of Swedish Snus, Nicotine Pouches and Other Tobacco Products with Oral Mucosal Health and Salivary Biomarkers. Dent. J. 2022, 10, 154. https://doi.org/10.3390/dj10080154
Miluna S, Melderis R, Briuka L, Skadins I, Broks R, Kroica J, Rostoka D. The Correlation of Swedish Snus, Nicotine Pouches and Other Tobacco Products with Oral Mucosal Health and Salivary Biomarkers. Dentistry Journal. 2022; 10(8):154. https://doi.org/10.3390/dj10080154
Chicago/Turabian StyleMiluna, Sintija, Ricards Melderis, Loreta Briuka, Ingus Skadins, Renars Broks, Juta Kroica, and Dagnija Rostoka. 2022. "The Correlation of Swedish Snus, Nicotine Pouches and Other Tobacco Products with Oral Mucosal Health and Salivary Biomarkers" Dentistry Journal 10, no. 8: 154. https://doi.org/10.3390/dj10080154
APA StyleMiluna, S., Melderis, R., Briuka, L., Skadins, I., Broks, R., Kroica, J., & Rostoka, D. (2022). The Correlation of Swedish Snus, Nicotine Pouches and Other Tobacco Products with Oral Mucosal Health and Salivary Biomarkers. Dentistry Journal, 10(8), 154. https://doi.org/10.3390/dj10080154