Effect of Commercial Children’s Mouthrinses and Toothpastes on the Viability of Neonatal Human Melanocytes: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
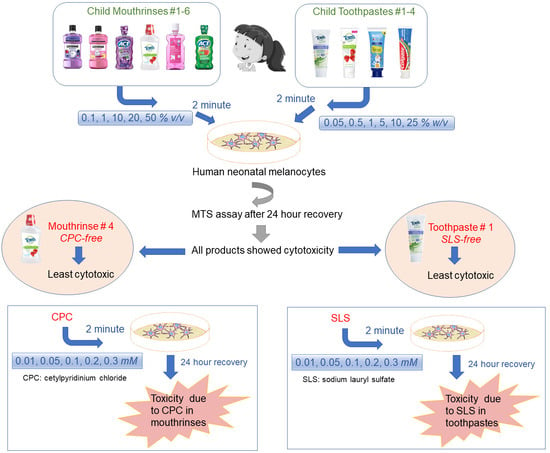
2.3. Preparation of Mouthrinses and Toothpaste-Conditioned Medium (TCM)
2.4. Cell Viability
2.5. Morphological Assessment
2.6. Statistical Analysis
3. Results
3.1. Exposure to Mouthrinses Significantly Lowered Cell Viability
3.2. Mouthrinses Treatment Altered Cell Morphology
3.3. Toothpaste Treatment Lowered Cell Viability
3.4. Toothpastes Treatment Altered Cell Morphology
3.5. CPC and SLS Treatment Lowered Cell Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bawaskar, H.S.; Bawaskar, P.H. Oral diseases: A global public health challenge. Lancet 2020, 395, 185–186. [Google Scholar] [PubMed]
- Li, Y.; Wang, W. Predicting caries in permanent teeth from caries in primary teeth: An eight-year cohort study. J. Dent. Res. 2002, 81, 561–566. [Google Scholar] [PubMed]
- Uribe, S.E.; Innes, N.; Maldupa, I. The global prevalence of early childhood caries: A systematic review with meta-analysis using the WHO diagnostic criteria. Int. J. Paediatr. Dent. 2021, 31, 817–830. [Google Scholar] [PubMed]
- Valkenburg, C.; Slot, D.E.; Bakker, E.W.; Van der Weijden, F.A. Does dentifrice use help to remove plaque? A systematic review. J. Clin. Periodontol. 2016, 43, 1050–1058. [Google Scholar] [PubMed]
- Nightingale, K.; Chinta, S.; Agarwal, P.; Nemelivsky, M.; Frisina, A.; Cao, Z.; Norman, R.; Fisch, G.; Corby, P. Toothbrush efficacy for plaque removal. Int. J. Dent. Hyg. 2014, 12, 251–256. [Google Scholar] [PubMed]
- Tellefsen, G.; Liljeborg, A.; Johannsen, A.; Johannsen, G. The role of the toothbrush in the abrasion process. Int. J. Dent. Hyg. 2011, 9, 284–290. [Google Scholar] [PubMed]
- Jongsma, M.A.; van de Lagemaat, M.; Busscher, H.J.; Geertsema-Doornbusch, G.I.; Atema-Smit, J.; van der Mei, H.C.; Ren, Y. Synergy of brushing mode and antibacterial use on in vivo biofilm formation. J. Dent. 2015, 43, 1580–1586. [Google Scholar]
- Sheiham, A.; Sabbah, W. Using universal patterns of caries for planning and evaluating dental care. Caries Res. 2010, 44, 141–150. [Google Scholar]
- Chikte, U.M.; Pochee, E.; Rudolph, M.J.; Reinach, S.G. Evaluation of stannous fluoride and chlorhexidine sprays on plaque and gingivitis in handicapped children. J. Clin. Periodontol. 1991, 18, 281–286. [Google Scholar]
- Mann, J.; Wolnerman, J.S.; Lavie, G.; Carlin, Y.; Garfunkel, A.A. Periodontal treatment needs and oral hygiene for institutionalized individuais with handicapping conditions. Spec. Care Dent. 1984, 4, 173–176. [Google Scholar]
- Alzahrani, M.M.; Bamashmous, S.; Alkharobi, H.; Alghamdi, A.; Alharbi, R.H.; Hassan, A.M.; Darwish, M.; Bukhari, A.; Mahmoud, A.B.; Alfaleh, M.A. Mouth rinses efficacy on salivary SARS-CoV-2 viral load: A randomized clinical trial. J. Med. Virol. 2023, 95, e28412. [Google Scholar] [PubMed]
- Muñoz-Basagoiti, J.; Perez-Zsolt, D.; León, R.; Blanc, V.; Raïch-Regué, D.; Cano-Sarabia, M.; Trinité, B.; Pradenas, E.; Blanco, J.; Gispert, J. Mouthwashes with CPC reduce the infectivity of SARS-CoV-2 variants in vitro. J. Dent. Res. 2021, 100, 1265–1272. [Google Scholar] [PubMed]
- Eduardo, F.D.P.; Corrêa, L.; Heller, D.; Daep, C.; Benitez, C.; Malheiros, Z.; Stewart, B.; Ryan, M.; Machado, C.; Hamerschlak, N. Salivary SARS-CoV-2 load reduction with mouthwash use: A randomized pilot clinical trial. Heliyon 2021, 7, e07346. [Google Scholar] [PubMed]
- Srikanth, R.; Shashikiran, N.; Reddy, V.S. Chocolate mouth rinse: Effect on plaque accumulation and mutans streptococci counts when used by children. J. Indian Soc. Pedod. Prev. Dent. 2008, 26, 67. [Google Scholar] [PubMed]
- Rirattanapong, P.; Rirattanapong, O. Concentrations of fluoride among commercially available mouthrinses for children in Thailand. Southeast Asian J. Trop. Med. Public Health 2019, 50, 411–415. [Google Scholar]
- Choudhari, S.; Gurunathan, D.; Kanthaswamy, A. Children’s perspective on color, smell and flavor of toothpaste. Indian J. Dent. Res. 2020, 31, 338. [Google Scholar] [PubMed]
- Sulaimon, S.S.; Kitchell, B.E. The biology of melanocytes. Vet. Dermatol. 2003, 14, 57–65. [Google Scholar] [PubMed]
- Natesan, S.C.; Ramakrishnan, B.P.; Krishnapillai, R.; Thomas, P. Biophysiology of oral mucosal melanocytes. J. Health Sci. Res. 2019, 10, 47–51. [Google Scholar]
- Feller, L.; Masilana, A.; Khammissa, R.A.; Altini, M.; Jadwat, Y.; Lemmer, J. Melanin: The biophysiology of oral melanocytes and physiological oral pigmentation. Head Face Med. 2014, 10, 8. [Google Scholar]
- Płonka, P.; Grabacka, M. Melanin synthesis in microorganisms: Biotechnological and medical aspects. Acta Biochim. Pol. 2006, 53, 429–443. [Google Scholar]
- Lu, Y.; Zhu, W.Y.; Tan, C.; Yu, G.H.; Gu, J.X. Melanocytes are potential immunocompetent cells: Evidence from recognition of immunological characteristics of cultured human melanocytes. Pigment Cell Res. 2002, 15, 454–460. [Google Scholar] [PubMed]
- Dummett, C.O. Physiologic pigmentation of the oral and cutaneous tissues in the Negro. J. Dent. Res. 1946, 25, 421–432. [Google Scholar] [PubMed]
- Stamatas, G.; Nikolovski, J.; Mack, M.; Kollias, N. Infant skin physiology and development during the first years of life: A review of recent findings based on in vivo studies. Int. J. Cosmet. Sci. 2011, 33, 17–24. [Google Scholar] [PubMed]
- Stamatas, G.N.; Nikolovski, J.; Luedtke, M.A.; Kollias, N.; Wiegand, B.C. Infant skin microstructure assessed in vivo differs from adult skin in organization and at the cellular level. Pediatr. Dermatol. 2010, 27, 125–131. [Google Scholar]
- Puizina Mladinic, E.; Puizina, J.; Gavic, L.; Tadin, A. Clinical Prospective Assessment of Genotoxic and Cytotoxic Effects of Fluoride Toothpaste and Mouthwash in Buccal Mucosal Cells. Biomedicines 2022, 10, 2206. [Google Scholar]
- Tadin, A.; Gavic, L.; Govic, T.; Galic, N.; Zorica Vladislavic, N.; Zeljezic, D. In vivo evaluation of fluoride and sodium lauryl sulphate in toothpaste on buccal epithelial cells toxicity. Acta Odontol. Scand. 2019, 77, 386–393. [Google Scholar]
- Rantanen, I.; Jutila, K.; Nicander, I.; Tenovuo, J.; Söderling, E. The effects of two sodium lauryl sulphate-containing toothpastes with and without betaine on human oral mucosa in vivo. Swed. Dent. J. 2003, 27, 31–34. [Google Scholar]
- Skaare, A.; Kjærheim, V.; Barkvoll, P.; Rølla, G. Skin reactions and irritation potential of four commercial toothpastes. Acta Odontol. Scand. 1997, 55, 133–136. [Google Scholar]
- Herlofson, B.B.; Barkvoll, P. The effect of two toothpaste detergents on the frequency of recurrent aphthous ulcers. Acta Odontol. Scand. 1996, 54, 150–153. [Google Scholar]
- Chahine, L.; Sempson, N.; Wagoner, C. The effect of sodium lauryl sulfate on recurrent aphthous ulcers: A clinical study. Compend. Contin. Educ. Dent. 1997, 18, 1238–1240. [Google Scholar]
- Herlofson, B.B.; Barkvoll, P. Sodium lauryl sulfate and recurrent aphthous ulcers: A preliminary study. Acta Odontol. Scand. 1994, 52, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.H.; Jafar, Z.J. In vitro cytotoxic effect of annona squamosa pulp extract as a mouthwash for children on human normal cell line. J. Baghdad Coll. Dent. 2022, 34, 60–66. [Google Scholar]
- Poggi, P.; Baena, R.R.Y.; Rizzo, S.; Rota, M.T. Mouthrinses with alcohol: Cytotoxic effects on human gingival fibroblasts in vitro. J. Periodontol. 2003, 74, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, M.H.; Mahounak, F.S.; Asgari, N.; Moradi, Z. Cytotoxicity of the ingredients of commonly used toothpastes and mouthwashes on human gingival fibroblasts. Front. Dent. 2019, 16, 450. [Google Scholar] [PubMed]
- Cvikl, B.; Lussi, A.; Moritz, A.; Gruber, R. Dentifrices for children differentially affect cell viability in vitro. Clin. Oral Investig. 2017, 21, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Pecci-Lloret, M.P.; López-García, S.; Rodríguez-Lozano, F.J.; Álvarez-Novoa, P.; García-Bernal, D. In vitro biocompatibility of several children’s toothpastes on human gingival fibroblasts. Int. J. Environ. Res. Public Health 2022, 19, 2954. [Google Scholar] [CrossRef] [PubMed]
- Birant, S.; Duran, Y.; Akkoc, T.; Seymen, F. Cytotoxic effects of different detergent containing children’s toothpastes on human gingival epithelial cells. BMC Oral Health 2022, 22, 66. [Google Scholar] [CrossRef]
- Birant, S.; Duran, Y.; Gokalp, M.; Akkoc, T.; Seymen, F. Effects of different detergent-containing children’s toothpastes on the viability, osteogenic and chondrogenic differentiation of human dental periodontal ligament stem cells and gingival stem cells in vitro. Tissue Cell 2021, 72, 101538. [Google Scholar]
- Eisen, D. Disorders of pigmentation in the oral cavity. Clin. Dermatol. 2000, 18, 579–587. [Google Scholar] [CrossRef]
- Nilima, S.; Vandana, K. Melanin: A scavenger in gingival inflammation. Indian J. Dent. Res. 2011, 22, 38. [Google Scholar]
- Human Epidermal Melanocytes, Neonatal, Lightly Pigmented Donor, (HEMn-LP). Available online: https://www.thermofisher.com/order/catalog/product/C0025C?SID=srch-srp-C0025C (accessed on 22 October 2023).
- Goenka, S. Biological Impact of the Ratio of E-Cigarette Liquid Base Constituents, Propylene Glycol and Vegetable Glycerin, on Primary Human Melanocytes. Oral 2023, 3, 40–56. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Effects of Fluoride Exposure on Primary Human Melanocytes from Dark and Light Skin. Toxics 2020, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S. In Vitro Evaluation of Dental Resin Monomers, Triethylene Glycol Dimethacrylate (TEGDMA), and 2-Hydroxyethyl Methacrylate (HEMA) in Primary Human Melanocytes: A Pilot Study. Oral 2023, 3, 353–371. [Google Scholar] [CrossRef]
- Goenka, S. Comparative study of Δ9-tetrahydrocannabinol and cannabidiol on melanogenesis in human epidermal melanocytes from different pigmentation phototypes: A pilot study. J. Xenobiot. 2022, 12, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Simon, S.R. A novel pro-melanogenic effect of standardized dry olive leaf extract on primary human melanocytes from lightly pigmented and moderately pigmented skin. Pharmaceuticals 2021, 14, 252. [Google Scholar] [CrossRef] [PubMed]
- Delijewski, M.; Wrześniok, D.; Beberok, A.; Rok, J.; Otręba, M.; Buszman, E. The effect of simultaneous exposure of HEMn-DP and HEMn-LP melanocytes to nicotine and UV-radiation on the cell viability and melanogenesis. Environ. Res. 2016, 151, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Otręba, M.; Beberok, A.; Wrześniok, D.; Buszman, E. In vitro melanogenesis inhibition by fluphenazine and prochlorperazine in normal human melanocytes lightly pigmented. DARU J. Pharm. Sci. 2018, 26, 85–89. [Google Scholar] [CrossRef]
- Goenka, S. Sepia melanin-loaded primary human gingival keratinocytes: An in vitro model for studies on pigmented gingiva. Oral 2023, 3, 254–265. [Google Scholar] [CrossRef]
- Qi, C.; Peng, X.; Yuan, S.; Zhang, M.; Xu, X.; Cheng, X. Evaluation of the Antibacterial and Anti-Inflammatory Effects of a Natural Products-Containing Toothpaste. Front. Cell. Infect. Microbiol. 2022, 98, 827643. [Google Scholar] [CrossRef]
- Honwad, S.; Ravi, S.; Donoghue, M.; Joshi, M. Immuno-histochemical and quantitative study of melanocytes and melanin granules in oral epithelial dysplasia. J. Clin. Diagn. Res. JCDR 2017, 11, ZC56. [Google Scholar] [CrossRef]
- Cantudo-Sanagustín, E.; Gutiérrez-Corrales, A.; Vigo-Martínez, M.; Serrera-Figallo, M.-Á.; Torres-Lagares, D.; Gutiérrez-Pérez, J.-L. Pathogenesis and clinicohistopathological characteristics of melanoacanthoma: A systematic review. J. Clin. Exp. Dent. 2016, 8, e327. [Google Scholar] [PubMed]
- Souza-Rodriguez, R.D.; Ferreira, S.D.S.; D’Almeida-Couto, R.S.; Lachowski, K.M.; Sobral, M.Â.P.; Marques, M.M. Choice of toothpaste for the elderly: An in vitro study. Braz. Oral Res. 2015, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cvikl, B.; Lussi, A.; Gruber, R. The in vitro impact of toothpaste extracts on cell viability. Eur. J. Oral Sci. 2015, 123, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Taddeo, F.; Medeiros, I.S.; Boaro, L.C.C.; Moreira, M.S.N.; Marques, M.M.; Calheiros, F.C. Relationship between toothpastes properties and patient-reported discomfort: Crossover study. Clin. Oral Investig. 2016, 20, 485–494. [Google Scholar] [CrossRef]
- Emmadi, P.; Ambalavanan, N.; Ramakrishnan, T.; Vijayalakshmi, R. Effect of three commercial mouth rinses on cultured human gingival fibroblast: An in vitro study. Indian J. Dent. Res. 2008, 19, 29. [Google Scholar]
- Schmidt, J.; Zyba, V.; Jung, K.; Rinke, S.; Haak, R.; Mausberg, R.F.; Ziebolz, D. Cytotoxic effects of octenidine mouth rinse on human fibroblasts and epithelial cells–an in vitro study. Drug Chem. Toxicol. 2016, 39, 322–330. [Google Scholar] [CrossRef]
- Barrett, A.; Scully, C. Human oral mucosal melanocytes: A review. J. Oral Pathol. Med. 1994, 23, 97–103. [Google Scholar] [CrossRef]
- Winterfeld, T.; Schlueter, N.; Harnacke, D.; Illig, J.; Margraf-Stiksrud, J.; Deinzer, R.; Ganss, C. Toothbrushing and flossing behaviour in young adults—A video observation. Clin. Oral Investig. 2015, 19, 851–858. [Google Scholar] [CrossRef]
- Gallagher, A.; Sowinski, J.; Bowman, J.; Barrett, K.; Lowe, S.; Patel, K.; Bosma, M.L.; Creeth, J.E. The effect of brushing time and dentifrice on dental plaque removal in vivo. Am. Dent. Hyg. Assoc. 2009, 83, 111–116. [Google Scholar]
- Tsourounakis, I.; Palaiologou-Gallis, A.A.; Stoute, D.; Maney, P.; Lallier, T.E. Effect of essential oil and chlorhexidine mouthwashes on gingival fibroblast survival and migration. J. Periodontol. 2013, 84, 1211–1220. [Google Scholar] [CrossRef]
- Lessa, F.C.R.; Aranha, A.M.F.; Nogueira, I.; Giro, E.M.A.; Hebling, J.; Costa, C.A.D.S. Toxicity of chlorhexidine on odontoblast-like cells. J. Appl. Oral Sci. 2010, 18, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, A.J.; Rumpf, D.A. Chlorhexidine-induced changes to human gingival fibroblast collagen and non-collagen protein production. J. Periodontol. 1999, 70, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Bean, T.A.; Zhuang, W.C.; Tong, P.Y.; Eick, J.D.; Chappelow, C.C.; Yourtee, D.M. Comparison of tetrazolium colorimetric and 51Cr release assays for cytotoxicity determination of dental biomaterials. Dent. Mater. 1995, 11, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Ghapanchi, J.; Kamali, F.; Moattari, A.; Poorshahidi, S.; Shahin, E.; Rezazadeh, F.; Khorshidi, H.; Jamshidi, S. In vitro comparison of cytotoxic and antibacterial effects of 16 commercial toothpastes. J. Int. Oral Health JIOH 2015, 7, 39. [Google Scholar] [PubMed]
- Torrado, A.; Valiente, M.; Zhang, W.; Li, Y.; Muñoz, C.A. Cytotoxicity of a new toothpaste based on an ion exchange resin mixture. Am. J. Dent. 2005, 18, 267–269. [Google Scholar] [PubMed]
- Sardari, F.; Arababadi, M.K.; Heiranizade, M.; Mosadeghi, M. Anti-inflammatory and cytotoxicity effects of Salvadora persica (meswak) extracts on jurkat t-cells. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 379. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ramirez, C.N.; Antczak, C.; Djaballah, H. Cell viability assessment: Toward content-rich platforms. Expert Opin. Drug Discov. 2010, 5, 223–233. [Google Scholar] [CrossRef]
- Zheng, Y.-B.; Meng, F.-G.; Chen, B.-Y.; Wang, X.-C. Inactivation and conformational changes of lactate dehydrogenase from porcine heart in sodium dodecyl sulfate solutions. Int. J. Biol. Macromol. 2002, 31, 97–102. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.; Inman, A.; Zhang, L. Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicol. Appl. Pharmacol. 2009, 234, 222–235. [Google Scholar] [CrossRef]
- Imai, K.; Kusakawa, S.; Tanoue, A.; Kuwagata, M.; Senuma, M.; Furuya, M.; Takashima, H. An attempt to cell recovery factor in cell differentiation culture with the embryonic stem cell test (EST). J. Oral Tissue Eng. 2009, 6, 152–158. [Google Scholar]
- Delbem, A.C.B.; Sassaki, K.T.; Castro, A.M.D.; Pinto, L.M.C.P.; Bergamaschi, M. Assement of the fluoride concentration and pH in different mouthrinses on the brazilian market. J. Appl. Oral Sci. 2003, 11, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Barkvoll, P.; Rölla, G. Triclosan protects the skin against dermatitis caused by sodium lauryl sulphate exposure. J. Clin. Periodontol. 1994, 21, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Waaler, S.M.; Rölla, G.; Skjörland, K.K.; Ögaard, B. Effects of oral rinsing with triclosan and sodium lauryl sulfate on dental plaque formation: A pilot study. Eur. J. Oral Sci. 1993, 101, 192–195. [Google Scholar] [CrossRef]
- Bednarkiewicz, A.; Rodrigues, R.M.; Whelan, M.P. Non-invasive monitoring of cytotoxicity based on kinetic changes of cellular autofluorescence. Toxicol. Vitr. 2011, 25, 2088–2094. [Google Scholar] [CrossRef]
- Deo, N.; Somasundaran, P.; Subramanyan, K.; Ananthapadmanabhan, K. Electron paramagnetic resonance study of the structure of lipid bilayers in the presence of sodium dodecyl sulfate. J. Colloid and Interface Sci. 2002, 256, 100–105. [Google Scholar] [CrossRef]
- Moore, C.; Addy, M.; Moran, J. Toothpaste detergents: A potential source of oral soft tissue damage? Int. J. Dent. Hyg. 2008, 6, 193–198. [Google Scholar] [CrossRef]
- Kim, M.; Lim, K.-M. Melanocytotoxic chemicals and their toxic mechanisms. Toxicol. Res. 2022, 38, 417–435. [Google Scholar] [CrossRef]
- Karaman, G.E.; Ünal, İ.; Beler, M.; Üstündağ, F.D.; Cansız, D.; Üstündağ, Ü.V.; Emekli-Alturfan, E.; Akyüz, S. Toothpastes for children and their detergent contents affect molecular mechanisms of odontogenesis in zebrafish embryos. Drug Chem. Toxicol. 2022. [Google Scholar] [CrossRef]
- Bennadi, D.; Kshetrimayum, N.; Sibyl, S.; Reddy, C. Toothpaste utilization profiles among preschool children. J. Clin. Diagn. Res. JCDR 2014, 8, 212. [Google Scholar] [CrossRef]
- Kahvecioğlu, F.; Ülker, H.E.; Tosun, G.; Özcan, M. Effect of pediatric toothpastes based on 500 to 1450 ppm sodium fluoride and amine fluoride with different detergents on oxidative stress and cell viability. Meandros Med. Dent. J. 2022, 23, 53–59. [Google Scholar] [CrossRef]
- Jang, S.-O.; Shim, Y.-S.; Choi, Y.-R. Evaluation of cytotoxicity of child toothpaste. Sci. Adv. Mater. 2016, 8, 331–335. [Google Scholar] [CrossRef]
- Creeth, J.; Bosma, M.L.; Govier, K. How much is a ‘pea-sized amount’? A study of dentifrice dosing by parents in three countries. Int. Dent. J. 2013, 63, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.M.; Maurice, T.J.; Jakobsen, J.R. A pilot study of preschoolers’ use of regular-flavored dentifrices and those flavored for children. Pediatr. Dent. 1992, 14, 388. [Google Scholar] [PubMed]
- Oliveira, M.J.L.; Paiva, S.M.; Martins, L.H.P.; Pordeus, I.A.; Lima, Y.B.; Cury, J.A. Influence of rinsing and expectoration after toothbrushing on fluoride dose and ingested amount by use of conventional and children’s fluoride dentifrices. Braz. Dent. J. 2006, 17, 100–105. [Google Scholar] [CrossRef] [PubMed]
- How to Keep Your Teeth Clean—NHS. Available online: https://www.nhs.uk/live-well/healthy-teeth-and-gums/how-to-keep-your-teeth-clean/#:~:text=After%20brushing%2C%20spit%20out%20any,and%20reduces%20its%20preventative%20effects (accessed on 20 October 2023).
- Hargreaves, J.; Ingram, G.; Wagg, B. Excretion studies on the ingestion of a monofluorophosphate toothpaste by children. Caries Res. 1970, 4, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Naccache, H.; Simard, P.L.; Trahan, L.; Brodeur, J.M.; Demers, M.; Lachapelle, D.; Bernard, P.M. Factors affecting the ingestion of fluoride dentifrice by children. J. Public Health Dent. 1992, 52, 222–226. [Google Scholar] [CrossRef]
- Reddy, B.A.; Ganapathy, D.; Kumar, P.K. Prevalence of toothpaste swallowing habit in children between the age group of 3 and 5 years. Drug Invent. Today 2019, 12, 1452–1455. [Google Scholar]
- Barnhart, W.E.; Hiller, L.K.; Leonard, G.J.; Michaels, S.E. Dentifrice usage and ingestion among four age groups. J. Dent. Res. 1974, 53, 1317–1322. [Google Scholar] [CrossRef]
- Brookes, Z.L.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef]
- Amini, P.; Araujo, M.W.B.; Wu, M.-M.; Charles, C.A.; Sharma, N.C. Comparative antiplaque and antigingivitis efficacy of three antiseptic mouthrinses: A two week randomized clinical trial. Braz. Oral Res. 2009, 23, 319–325. [Google Scholar] [CrossRef]
- Retamal-Valdes, B.; Soares, G.M.; Stewart, B.; Figueiredo, L.C.; Faveri, M.; Miller, S.; Zhang, Y.P.; Feres, M. Effectiveness of a pre-procedural mouthwash in reducing bacteria in dental aerosols: Randomized clinical trial. Braz. Oral Res. 2017, 31, 21. [Google Scholar] [CrossRef] [PubMed]
- Setiawatie, E.M.; Valentina, R.; Meiliana, R.S. Effectiveness of cetylpyridinium chloride in reducing the growth of bacteria that cause periodontal disease. e-GiGi 2023, 11, 115–120. [Google Scholar] [CrossRef]
- Van Strydonck, D.A.; Slot, D.E.; Van der Velden, U.; Van der Weijden, F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: A systematic review. J. Clin. Periodontol. 2012, 39, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Haps, S.; Slot, D.; Berchier, C.; Van der Weijden, G. The effect of cetylpyridinium chloride-containing mouth rinses as adjuncts to toothbrushing on plaque and parameters of gingival inflammation: A systematic review. Int. J. Dent. Hyg. 2008, 6, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Auer, D.L.; Buchalla, W.; Hiller, K.-A.; Maisch, T.; Hellwig, E.; Al-Ahmad, A.; Cieplik, F. Cetylpyridinium chloride: Mechanism of action, antimicrobial efficacy in biofilms, and potential risks of resistance. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef]
- Costa, X.; Laguna, E.; Herrera, D.; Serrano, J.; Alonso, B.; Sanz, M. Efficacy of a new mouth rinse formulation based on 0.07% cetylpyridinium chloride in the control of plaque and gingivitis: A 6-month randomized clinical trial. J. Clin. Periodontol. 2013, 40, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, M.; Rosema, N.; Versteeg, P.; Slot, D.; Van Winkelhoff, A.; Van der Weijden, G. Long-term efficacy of a 0.07% cetylpyridinium chloride mouth rinse in relation to plaque and gingivitis: A 6-month randomized, vehicle-controlled clinical trial. Int. J. Dent. Hyg. 2015, 13, 93–103. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, J.; Lim, Y.M.; Kim, E.J.; Yoon, B.I.; Kim, P.; Yu, S.D.; Eom, I.C.; Shim, I. Comprehensive pulmonary toxicity assessment of cetylpyridinium chloride using A549 cells and Sprague–Dawley rats. J. Appl. Toxicol. 2021, 41, 470–482. [Google Scholar] [CrossRef]
- Kano, S.; Sugibayashi, K. Kinetic analysis on the skin disposition of cytotoxicity as an index of skin irritation produced by cetylpyridinium chloride: Comparison of in vitro data using a three-dimensional cultured human skin model with in vivo results in hairless mice. Pharm. Res. 2006, 23, 329–335. [Google Scholar] [CrossRef]
- Qiu, X.; Tengbe, M.S.; Xia, X.; Dong, K.; Chen, C.; Shi, Y.; Li, M.; Xu, H.; Wu, X.; Chen, K. Impacts of cetylpyridinium chloride on the survival, development, behavior, and oxidative stress of early-life-stage zebrafish (Danio rerio). Antioxidants 2022, 11, 676. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Chatterjee, A.; Chatterjee, S.; Saha, N.C. Commonly used surfactants sodium dodecyl sulphate, cetylpyridinium chloride and sodium laureth sulphate and their effects on antioxidant defence system and oxidative stress indices in Cyprinus carpio L.: An integrated in silico and in vivo approach. Environ. Sci. Pollut. Res. 2022, 29, 30622–30637. [Google Scholar] [CrossRef] [PubMed]
- Chávez, E.; Bravo, C. Anisotropic action of cetyl pyridinium chloride on rat heart mitochondria. Arch. Biochem. Biophys. 1982, 213, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; He, G.; Tomilov, A.; Sahdeo, S.; Denison, M.S.; Cortopassi, G. In vitro evaluation of mitochondrial function and estrogen signaling in cell lines exposed to the antiseptic cetylpyridinium chloride. Environ. Health Perspect. 2017, 125, 087015. [Google Scholar] [CrossRef] [PubMed]
- Ülker, M.; Çelik, A.; Yavuz, E.; Kahvecioğlu, F.; Ülker, H.E. Real-time analysis of antiproliferative effects of mouthwashes containing alcohol, sodium fluoride, cetylpyridinium chloride, and chlorhexidine in vitro. BioMed Res. Int. 2021, 2021, 2610122. [Google Scholar] [CrossRef] [PubMed]
- Song, I.-S.; Lee, J.E.; Park, J.-B. The effects of various mouthwashes on osteoblast precursor cells. Open Life Sci. 2019, 14, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Soames, J. The morphology and quantitative distribution of dopa-positive melanocytes in the gingival epithelium of Caucasians. Oral Surg. Oral Med. Oral Pathol. 1974, 38, 254–258. [Google Scholar] [CrossRef]
- Barrett, A.; Raja, A. The immunohistochemical identification of human oral mucosal melanocytes. Arch. Oral Biol. 1997, 42, 77–81. [Google Scholar] [CrossRef]
- Nakamura, M.; Ueda, Y.; Hayashi, M.; Kato, H.; Furuhashi, T.; Morita, A. Tobacco smoke–induced skin pigmentation is mediated by the aryl hydrocarbon receptor. Exp. Dermatol. 2013, 22, 556–558. [Google Scholar] [CrossRef]
- Hedin, C.A.; Larsson, Å. The ultrastructure of the gingival epithelium in smokers’ melanosis. J. Periodontal Res. 1984, 19, 177–190. [Google Scholar]
- Eaturi, E.; Reddy, K.; Avula, H.; Bolla, V.; Mishra, A. Evaluation of prevalence and severity of gingival pigmentation and its correlation with skin complexion. Sch. Acad. J. Biosci. 2017, 5, 596–610. [Google Scholar]
- Szabó, G. Quantitative histological investigations on the melanocyte system of the human epidermis. Pigment Cell Biol. 1959, 99–125. [Google Scholar]
- Hanioka, T.; Tanaka, K.; Ojima, M.; Yuuki, K. Association of melanin pigmentation in the gingiva of children with parents who smoke. Pediatrics 2005, 116, e186–e190. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Ganiger, K.; Satyanarayana, A.; Rahul, A.; Shetty, S. Effect of environmental tobacco smoke from smoker parents on gingival pigmentation in children and young adults: A cross-sectional study. J. Periodontol. 2011, 82, 956–962. [Google Scholar] [CrossRef]
- Kim, N.S.; Kang, W.; Cho, J. Behavioral differences between donor site-matched adult and neonatal melanocytes in culture. Arch. Dermatol. Res. 2000, 292, 233–239. [Google Scholar] [CrossRef]
- Glimcher, M.E.; Kostick, R.M.; Szabo, G. The epidermal melanocyte system in newborn human skin. A quantitative histologic study. J. Investig. Dermatol. 1973, 61, 344–347. [Google Scholar] [CrossRef]
- Gilchrest, B.A.; Vrabel, M.A.; Flynn, E.; Szabo, G. Selective cultivation of human melanocytes from newborn and adult epidermis. J. Investig. Dermatol. 1984, 83, 370–376. [Google Scholar] [CrossRef]
- Eun, H.; Chung, J.; Jung, S.; Cho, K.; Kim, K. A comparative study of the cytotoxicity of skin irritants on cultured human oral and skin keratinocytes. Br. J. Dermatol. 1994, 130, 24–28. [Google Scholar] [CrossRef]
- Mattei, B.M.; Imanishi, S.A.; de Oliveira Ramos, G.; de Campos, P.S.; Weiss, S.G.; Deliberador, T.M. Mouthwash with active oxygen (Blue® M) induces keratinocytes proliferation. Open J. Stomatol. 2020, 10, 107. [Google Scholar] [CrossRef]
- Aydın, N.; Karaoğlanoğlu, S.; Oktay, E.A.; Süloğlu, A.K. Cytotoxic effects of bulk-fill composites on L929 fibroblast cells. Braz. Dent. Sci. 2021, 24, 1–9. [Google Scholar] [CrossRef]
- Chaves, C.D.A.L.; de Souza Costa, C.A.; Vergani, C.E.; Chaves de Souza, P.P.; Machado, A.L. Effects of soft denture liners on L929 fibroblasts, HaCaT keratinocytes, and RAW 264.7 macrophages. BioMed Res. Int. 2014, 2014, 840613. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Kim, M.-J.; Kwon, J.-S.; Lee, S.-B.; Kim, K.-M. Cytotoxicity of light-cured dental materials according to different sample preparation methods. Materials 2017, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Moharamzadeh, K.; Van Noort, R.; Brook, I.M.; Scutt, A.M. Cytotoxicity of resin monomers on human gingival fibroblasts and HaCaT keratinocytes. Dent. Mater. 2007, 23, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Saw, T.Y.; Cao, T.; Yap, A.U.J.; Ng, M.M.L. Tooth slice organ culture and established cell line culture models for cytotoxicity assessment of dental materials. Toxicol. Vitr. 2005, 19, 145–154. [Google Scholar] [CrossRef]
- Sforna, M.; Chiaradia, E.; Porcellato, I.; Silvestri, S.; Moretti, G.; Mechelli, L.; Brachelente, C. Characterization of Primary Cultures of Normal and Neoplastic Canine Melanocytes. Animals 2021, 11, 768. [Google Scholar] [CrossRef]
- Tang, J.; Li, Q.; Cheng, B.; Jing, L. Primary culture of human face skin melanocytes for the study of hyperpigmentation. Cytotechnology 2014, 66, 891–898. [Google Scholar] [CrossRef]
- Yohn, J.J.; Lyons, M.B.; Norris, D.A. Cultured human melanocytes from black and white donors have different sunlight and ultraviolet A radiation sensitivities. J. Investig. Dermatol. 1992, 99, 454–459. [Google Scholar] [CrossRef] [PubMed]






| Mouthrinse # | Mouthrinse | Distributor | Active Ingredient | Inactive Ingredients |
|---|---|---|---|---|
| 1 | Listerine, Berry Splash | Johnson & Johnson Consumer Inc. (Skillman, NJ, USA) | Stannous fluoride 0.02% (0.01% w/v fluoride ion) | Water, sorbitol, flavor, phosphoric acid, cetylpyridinium chloride, sucralose, sodium saccharin, disodium phosphate, red 33, green 3 |
| 2 | Listerine, Pink Lemonade | Johnson & Johnson Consumer Inc. (Skillman, NJ, USA) | Sodium fluoride 0.02% (0.01% w/v fluoride ion) | Water, sorbitol, flavor, phosphoric acid, cetylpyridinium chloride, sucralose, sodium saccharin, disodium phosphate, red 33, red 40 |
| 3 | ACT Kids, Groovy Grape | Chattem, Inc., a Sanofi Company (Chattanooga, TN, USA) | Sodium fluoride 0.02% (0.01% w/v fluoride ion) | Water, sorbitol, flavor, cetylpyridinium chloride, sucralose, disodium phosphate, red 33, polysorbate 20, poloxamer 407, propylene glycol, benzyl alcohol, blue 1, calcium disodium EDTA, sodium benzoate, sodium phosphate, potassium sorbate |
| 4 | Tom’s of Maine, Strawberry | Tom’s of Maine, Inc. (Kennebunk, ME, USA) | Sodium fluoride 0.04% (0.02% w/v fluoride ion) | Water, glycerin, Aloe barbadensis leaf juice, xylitol, sodium phosphate, propanediol, benzoic acid, natural flavor, phosphoric acid, menthol, Fragaria ananassa (strawberry) fruit juice, Ananas sativus (pineapple) fruit juice, Citrus aurantium dulcis (Orange) juice, Citrus limon (Lemon) juice, Mangifera indica (Mango) juice, Rebaudioside A |
| 5 | Kids’ Anticavity, Bubblegum | Target Corp. (Mpls, MN, USA) | Sodium fluoride 0.05% (0.02% w/v fluoride ion) | Water, sorbitol, flavor, cetylpyridinium chloride, disodium phosphate, red 33, polysorbate 80, poloxamer 407, propylene glycol, benzyl alcohol, calcium disodium EDTA, sodium benzoate, sodium phosphate, disodium EDTA, sodium saccharin |
| 6 | ACT Kids, Wild Watermelon | Chattem, Inc., a Sanofi Company (Chattanooga, TN, USA) | Sodium fluoride 0.05% (0.02% w/v fluoride ion) | Water, sorbitol, flavor, cetylpyridinium chloride, sucralose, disodium phosphate, yellow 5, green 3, polysorbate 20, poloxamer 407, propylene glycol, calcium disodium EDTA, sodium benzoate, sodium phosphate, potassium sorbate |
| Toothpaste # | Name | Distributor | Active Ingredient | Inactive Ingredients |
|---|---|---|---|---|
| 1 | Tom’s of Maine, Toddler Toothpaste | Tom’s of Maine Inc., Kennebunk, ME 04043, USA | Fluoride free | Water, hydrated silica, glycerin, propanediol, xylitol, benzyl alcohol, citric acid, natural flavor, carrageenan |
| 2 | Tom’s of Maine, Silly Strawberry | Tom’s of Maine Inc., Kennebunk, ME 04043, USA | Fluoride free | Water, hydrated silica, glycerin, benzyl alcohol, calcium carbonate, sodium lauryl sulfate, natural flavor, carrageenan, Citrus limon (lemon) juice, Mangifera indica (mango) juice, Citrus aurantium dulcis (orange) juice, Ananas sativus (pineapple) fruit juice, Fragaria ananassa (strawberry) fruit juice |
| 3 | Crest Mystic (3+ years), Magical Bubblegum | Procter & Gamble, Cincinnati, OH, 45202, USA | Sodium fluoride 0.243% (0.15% w/v fluoride ion) | Water, sorbitol, hydrated silica, cellulose gum, sodium lauryl sulfate, flavor, sodium saccharin, trisodium phosphate, sodium phosphate, carbomer, red 40 |
| 4 | Colgate Fluoride cavity protection (kids), Bubble fruit® | Colgate-Palmolive Company, NY, 10022, USA | Sodium fluoride 0.24% (0.15% w/v fluoride ion) | Water, sorbitol, hydrated silica, cellulose gum, sodium lauryl sulfate, flavor, sodium saccharin, PEG-12, blue 1, yellow-10 |
| Mouthrinse | IC50 (% v/v) |
|---|---|
| #1 | 2.48 ± 1.04 |
| #2 | 2.16 ± 1.03 |
| #3 | 6.06 ± 1.97 |
| #4 | n.d. |
| #5 | 1.96 ± 0.39 |
| #6 | 6.20 ± 1.11 |
| Toothpaste | IC50 (% w/v) |
|---|---|
| #1 | n.d. |
| #2 | 1.25 ± 0.27 |
| #3 | 0.62 ± 0.17 |
| #4 | 1.60 ± 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goenka, S.; Lee, H.-M. Effect of Commercial Children’s Mouthrinses and Toothpastes on the Viability of Neonatal Human Melanocytes: An In Vitro Study. Dent. J. 2023, 11, 287. https://doi.org/10.3390/dj11120287
Goenka S, Lee H-M. Effect of Commercial Children’s Mouthrinses and Toothpastes on the Viability of Neonatal Human Melanocytes: An In Vitro Study. Dentistry Journal. 2023; 11(12):287. https://doi.org/10.3390/dj11120287
Chicago/Turabian StyleGoenka, Shilpi, and Hsi-Ming Lee. 2023. "Effect of Commercial Children’s Mouthrinses and Toothpastes on the Viability of Neonatal Human Melanocytes: An In Vitro Study" Dentistry Journal 11, no. 12: 287. https://doi.org/10.3390/dj11120287
APA StyleGoenka, S., & Lee, H. -M. (2023). Effect of Commercial Children’s Mouthrinses and Toothpastes on the Viability of Neonatal Human Melanocytes: An In Vitro Study. Dentistry Journal, 11(12), 287. https://doi.org/10.3390/dj11120287